当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
para‐Quinone Methides as Acceptors in 1,6‐Nucleophilic Conjugate Addition Reactions for the Synthesis of Structurally Diverse Molecules
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1002/ejoc.201901796 Carolina G. S. Lima 1 , Fernanda P. Pauli 1 , Dora C. S. Costa 1 , Acácio S. de Souza 1 , Luana S. M. Forezi 1 , Vitor F. Ferreira 2 , Fernando de Carvalho da Silva 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1002/ejoc.201901796 Carolina G. S. Lima 1 , Fernanda P. Pauli 1 , Dora C. S. Costa 1 , Acácio S. de Souza 1 , Luana S. M. Forezi 1 , Vitor F. Ferreira 2 , Fernando de Carvalho da Silva 1
Affiliation
|
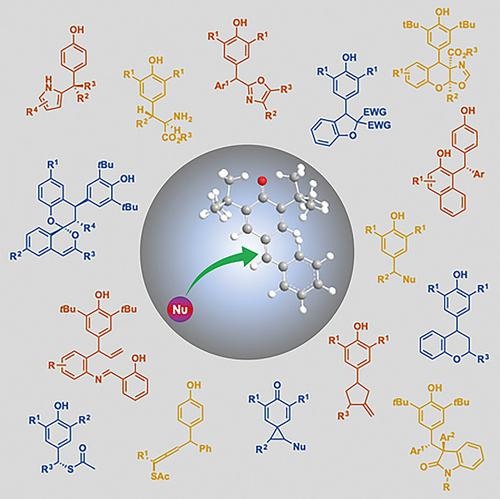
para‐Quinone methides (p‐QMs) are naturally occurring molecules with two electronically different exocyclic conjugate substituents in their structure, carbonyl and methylidene, which gives them a pronounced reactivity owing to the polarization of the molecule. By attack of nucleophiles in the terminal carbon exocyclic double bond, they generate 1,6‐addition products.

中文翻译:

对苯二甲腈作为1,6-亲核缀合物加成反应中的受体,用于合成结构多样的分子
对-醌甲基化物(p- QMs)是天然存在的分子,其结构中具有两个电子不同的环外共轭取代基,即羰基和亚甲基,由于分子的极化,它们具有显着的反应性。通过末端碳外环双键中亲核试剂的攻击,它们会生成1,6加成产物。

更新日期:2020-03-31

中文翻译:

对苯二甲腈作为1,6-亲核缀合物加成反应中的受体,用于合成结构多样的分子
对-醌甲基化物(p- QMs)是天然存在的分子,其结构中具有两个电子不同的环外共轭取代基,即羰基和亚甲基,由于分子的极化,它们具有显着的反应性。通过末端碳外环双键中亲核试剂的攻击,它们会生成1,6加成产物。




























 京公网安备 11010802027423号
京公网安备 11010802027423号