当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular bismacycles for the selective C-H arylation of phenols and naphthols.
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-02-27 , DOI: 10.1038/s41557-020-0425-4 Mark Jurrat 1, 2 , Lorenzo Maggi 1, 2 , William Lewis 2, 3 , Liam T Ball 1, 2
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-02-27 , DOI: 10.1038/s41557-020-0425-4 Mark Jurrat 1, 2 , Lorenzo Maggi 1, 2 , William Lewis 2, 3 , Liam T Ball 1, 2
Affiliation
|
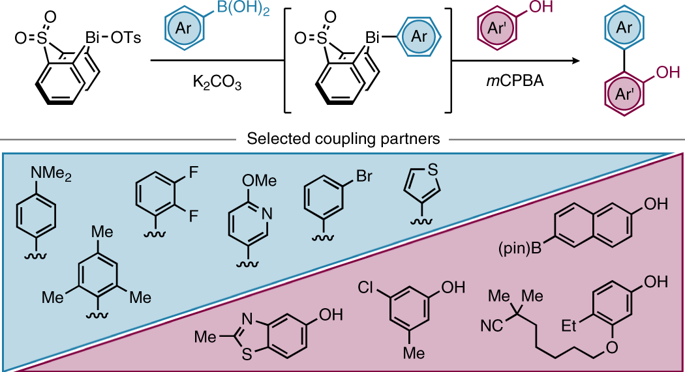
Given the important role played by 2-hydroxybiaryls in organic, medicinal and materials chemistry, concise methods for the synthesis of this common motif are extremely valuable. In seeking to extend the lexicon of synthetic chemists in this regard, we have developed an expedient and general strategy for the ortho-arylation of phenols and naphthols using readily available boronic acids. Our methodology relies on in situ generation of a uniquely reactive Bi(V) arylating agent from a bench-stable Bi(III) precursor via telescoped B-to-Bi transmetallation and oxidation. By exploiting reactivity that is orthogonal to conventional metal-catalysed manifolds, diverse aryl and heteroaryl partners can be rapidly coupled to phenols and naphthols under mild conditions. Following arylation, high-yielding recovery of the Bi(III) precursor allows for its efficient re-use in subsequent reactions. Mechanistic interrogation of each key step of the methodology informs its practical application and provides fundamental insight into the underexploited reactivity of organobismuth compounds.
中文翻译:

用于苯酚和萘酚的选择性 CH 芳基化的模块化双环。
鉴于 2-羟基联芳基化合物在有机化学、药物化学和材料化学中的重要作用,合成这种常见基序的简明方法非常有价值。在寻求扩展合成化学家在这方面的词典时,我们开发了一种使用现成的硼酸对苯酚和萘酚进行邻位芳基化的权宜之计和通用策略。我们的方法依赖于通过伸缩的 B 到 Bi 转金属和氧化从工作台稳定的 Bi(III) 前体原位生成独特的反应性 Bi(V) 芳基化剂。通过利用与传统金属催化歧管正交的反应性,多种芳基和杂芳基伙伴可以在温和条件下与苯酚和萘酚快速偶联。芳基化后,Bi(III)前体的高产率回收允许其在后续反应中有效地重复使用。对该方法的每个关键步骤的机械询问为其实际应用提供了信息,并提供了对有机铋化合物未充分利用的反应性的基本见解。
更新日期:2020-02-27
中文翻译:

用于苯酚和萘酚的选择性 CH 芳基化的模块化双环。
鉴于 2-羟基联芳基化合物在有机化学、药物化学和材料化学中的重要作用,合成这种常见基序的简明方法非常有价值。在寻求扩展合成化学家在这方面的词典时,我们开发了一种使用现成的硼酸对苯酚和萘酚进行邻位芳基化的权宜之计和通用策略。我们的方法依赖于通过伸缩的 B 到 Bi 转金属和氧化从工作台稳定的 Bi(III) 前体原位生成独特的反应性 Bi(V) 芳基化剂。通过利用与传统金属催化歧管正交的反应性,多种芳基和杂芳基伙伴可以在温和条件下与苯酚和萘酚快速偶联。芳基化后,Bi(III)前体的高产率回收允许其在后续反应中有效地重复使用。对该方法的每个关键步骤的机械询问为其实际应用提供了信息,并提供了对有机铋化合物未充分利用的反应性的基本见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号