Separation and Purification Technology ( IF 8.6 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.seppur.2020.116767 Ziying Wang , Na An , Yisheng Shao , Naiyun Gao , Erdeng Du , Bin Xu
|
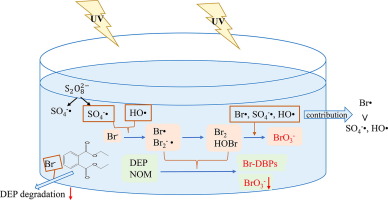
Bromide (Br-), which is omnipresent in water bodies, can not only affect the degradation kinetics of target pollutants but may also form undesired byproducts (such as bromate () and brominated disinfection byproducts (Br-DBPs)) through sulfate radical ()-based advanced oxidation processes (AOPs). This study found that Br- significantly suppressed the degradation of diethyl phthalate (DEP) based on the ultraviolet (UV)/persulfate (PS) treatment and was efficiently converted into reactive species (radicals and free bromine) by the or hydroxyl radical (HO•) generated in the UV/PS system. These reactive bromine species could, in turn, brominate the phenolic degradation intermediates of DEP as well as natural organic matter (NOM), yielding Br-DBPs, including tribromomethane (TBM), which was observed when Br-, DEP and/or NOM coexisted in this UV/PS system. However, the Br-DBPs were a short-lived form of bromine during the transformation of Br– and were degraded by the excessive oxidants (e.g., and HO•). Instead, Br- was eventually transformed into , with free bromine acting as the requisite intermediate. The formation initially showed a delay before increasing monotonically. In general, raising the PS dosage and the initial Br- concentrations enhanced both the maximum concentration of TBM and the formation of , while increasing the amounts of DEP and NOM facilitated the former and inhibited the latter. The maximum concentration of TBM increased while the formation of was suppressed with increasing pH from 5.0 to 8.0. In addition, through simulating the steady-state concentration of radicals, it was found that the contribution of bromine atom radical (Br•) towards oxidizing free bromine to was far greater than those of and HO•. The findings demonstrate the potential negative effects of Br- on -based AOPs, which need to be considered when this technology is applied in practice.
中文翻译:

在溴化物存在下进行UV /过硫酸盐处理的实验和模拟研究:对降解动力学,溴化消毒副产物和溴酸盐形成的影响
溴(溴- ),这是在水体无所不在,不仅可以影响目标污染物的降解动力学,但也可形成不需要的副产物(如溴酸盐()和通过硫酸根的溴化消毒副产物(Br-DBPs)()为基础的高级氧化工艺(AOP)。本研究发现,溴-显著基于紫外线(UV)/过硫酸盐(PS)治疗抑制邻苯二甲酸二乙酯(DEP)的降解,并有效地转换成由所述反应性物质(自由基和游离溴)或在UV / PS系统中产生的羟基自由基(HO •)。这些反应性溴物种可以反过来,溴化DEP的酚降解中间体以及天然有机物质(NOM),得到BR-消毒副产物,包括三溴甲烷(TBM),其中观察到当溴-,DEP和/或NOM共存在此UV / PS系统中。然而,BR-消毒副产物如下:Br的变换期间溴的短命形式-和由过度的氧化剂(例如被降解,和HO •)。相反,溴-最终被转化成,其中游离溴是必需的中间体。的形成最初显示出延迟,然后单调增加。一般情况下,提高了PS的剂量和初始溴-浓度增强二者TBM的最大浓度而形成的,而增加DEP和NOM的量则促进前者并抑制后者。TBM的最大浓度在形成的同时增加。pH值从5.0增加到8.0时,抑制作用被抑制。另外,通过模拟自由基的稳态浓度,发现溴原子自由基(Br •)对氧化游离溴为 远远大于 和HO •。该发现证明BR的潜在负面影响-上的AOP,在实际应用此技术时需要考虑。

























 京公网安备 11010802027423号
京公网安备 11010802027423号