当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The adsorption and dissociation of N2O on CuO(111) Surface: The Effect of surface structures
Surface Science ( IF 1.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.susc.2020.121596 Wei Suo , Shujuan Sun , Ningning Liu , Xiaojun Li , Yanji Wang
Surface Science ( IF 1.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.susc.2020.121596 Wei Suo , Shujuan Sun , Ningning Liu , Xiaojun Li , Yanji Wang
|
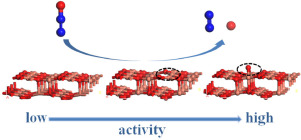
Abstract The adsorption and dissociation of N2O on the perfect, oxygen-deficient and oxygen-precovered CuO(111) surfaces are investigated with density functional theory (DFT) generalized gradient approximation. By calculating and comparing the adsorption energy, geometric configuration parameters and Mulliken charges at different adsorption sites, the most stable adsorption configurations have been determined. Possible reaction pathways of N2O on different kinds of CuO(111) surfaces are proposed, which include the N N bond cleavage and N O bond cleavage. On the perfect surface, the energy barriers of N2O dissociated into N2, O atom and NO, N atom are 261.65 and 526.24 kJ mol−1, respectively. Compared with the dissociation of N2O on the perfect surface, the reaction energy barriers for N2O dissociation on the oxygen-deficient and pre-covered oxygen surface are obviously reduced. The result implies that the presence of oxygen vacancy and pre-covered oxygen can significantly promote the dissociation of N2O.
中文翻译:

N2O在CuO(111)表面的吸附和解离:表面结构的影响
摘要 利用密度泛函理论 (DFT) 广义梯度近似研究了 N2O 在完美、缺氧和氧预覆盖的 CuO(111) 表面上的吸附和解离。通过计算和比较不同吸附位点的吸附能、几何构型参数和马利肯电荷,确定了最稳定的吸附构型。提出了 N2O 在不同种类的 CuO(111) 表面上可能的反应途径,包括 NN 键断裂和 NO 键断裂。在完美表面上,N2O 分解为 N2、O 原子和 NO、N 原子的能垒分别为 261.65 和 526.24 kJ mol-1。与 N2O 在完美表面的解离相比,在缺氧和预覆盖的氧气表面上,N2O 解离的反应能垒明显降低。结果表明氧空位和预覆盖氧的存在可以显着促进 N2O 的解离。
更新日期:2020-06-01
中文翻译:

N2O在CuO(111)表面的吸附和解离:表面结构的影响
摘要 利用密度泛函理论 (DFT) 广义梯度近似研究了 N2O 在完美、缺氧和氧预覆盖的 CuO(111) 表面上的吸附和解离。通过计算和比较不同吸附位点的吸附能、几何构型参数和马利肯电荷,确定了最稳定的吸附构型。提出了 N2O 在不同种类的 CuO(111) 表面上可能的反应途径,包括 NN 键断裂和 NO 键断裂。在完美表面上,N2O 分解为 N2、O 原子和 NO、N 原子的能垒分别为 261.65 和 526.24 kJ mol-1。与 N2O 在完美表面的解离相比,在缺氧和预覆盖的氧气表面上,N2O 解离的反应能垒明显降低。结果表明氧空位和预覆盖氧的存在可以显着促进 N2O 的解离。


























 京公网安备 11010802027423号
京公网安备 11010802027423号