当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microfluidics for Personalized Reactions to Demonstrate Stoichiometry
Journal of Chemical Education ( IF 3 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.jchemed.9b00544 Lindsay M. Veltri 1 , Lisa A. Holland 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.jchemed.9b00544 Lindsay M. Veltri 1 , Lisa A. Holland 1
Affiliation
|
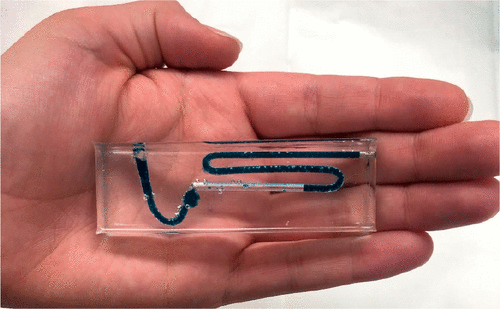
Stoichiometry, the ideal gas law, and the concept of limiting reagent are challenging principles for students to conceptualize in introductory chemistry. These topics are fundamental to successfully mastering other chemistry content. Therefore, a handheld microfluidic device was designed as a new tool to visualize these principles. Using food-grade baking soda, vinegar, and carbonated water as reagents, the students were engaged in a hands-on learning experience. The baking soda and vinegar were the source of sodium bicarbonate and acetic acid which were combined in a 1:1 mol ratio to form carbon dioxide gas which was then quantified using the device, thereby illustrating the ideal gas law. Experiments were devised to support learning outcomes that involved using the ideal gas law to interconvert moles and volume, balancing chemical equations, quantifying the amount of product generated from a known amount of reactant, conveying measurement uncertainty, and postulating sources of experimental error. The microfluidic device is a fast and cost-effective tool to teach stoichiometry. Moreover, the use of food-grade reagents makes the activity accessible as well as safe enough to be conducted outside of a traditional laboratory setting.
中文翻译:

微流体用于个性化反应以显示化学计量
化学计量学,理想气体定律和限制试剂的概念对学生来说是概论入门化学的挑战性原理。这些主题是成功掌握其他化学内容的基础。因此,手持式微流体设备被设计为可视化这些原理的新工具。学生使用食品级小苏打,醋和碳酸水作为试剂,进行了动手学习的体验。小苏打和醋是碳酸氢钠和乙酸的来源,它们以1:1的摩尔比混合形成二氧化碳气体,然后使用该设备对其进行定量,从而说明了理想的气体定律。设计实验以支持学习成果,其中涉及使用理想气体定律相互转化摩尔和体积,平衡化学方程式,量化从已知量的反应物产生的产物的量,传达测量不确定性,并假设实验误差的来源。微流体装置是教授化学计量学的快速且成本有效的工具。此外,食品级试剂的使用使得该活动可访问且安全,足以在传统实验室环境之外进行。
更新日期:2020-02-27
中文翻译:

微流体用于个性化反应以显示化学计量
化学计量学,理想气体定律和限制试剂的概念对学生来说是概论入门化学的挑战性原理。这些主题是成功掌握其他化学内容的基础。因此,手持式微流体设备被设计为可视化这些原理的新工具。学生使用食品级小苏打,醋和碳酸水作为试剂,进行了动手学习的体验。小苏打和醋是碳酸氢钠和乙酸的来源,它们以1:1的摩尔比混合形成二氧化碳气体,然后使用该设备对其进行定量,从而说明了理想的气体定律。设计实验以支持学习成果,其中涉及使用理想气体定律相互转化摩尔和体积,平衡化学方程式,量化从已知量的反应物产生的产物的量,传达测量不确定性,并假设实验误差的来源。微流体装置是教授化学计量学的快速且成本有效的工具。此外,食品级试剂的使用使得该活动可访问且安全,足以在传统实验室环境之外进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号