当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy.
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.jconrel.2020.02.040 Qianwen Yu 1 , Yue Qiu 1 , Jianping Li 1 , Xian Tang 1 , Xuhui Wang 1 , Xingli Cun 1 , Shanshan Xu 1 , Yayuan Liu 1 , Man Li 1 , Zhirong Zhang 1 , Qin He 1
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.jconrel.2020.02.040 Qianwen Yu 1 , Yue Qiu 1 , Jianping Li 1 , Xian Tang 1 , Xuhui Wang 1 , Xingli Cun 1 , Shanshan Xu 1 , Yayuan Liu 1 , Man Li 1 , Zhirong Zhang 1 , Qin He 1
Affiliation
|
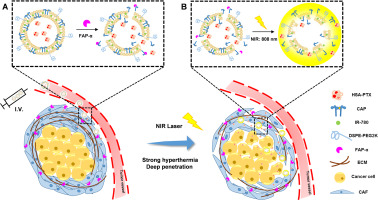
Pancreatic ductal adenocarcinoma (PDAC) is rich in cancer-associated fibroblasts (CAFs), which participate in the formation of tumor stroma. However, the dense tumor stroma of PDAC presents major barriers to drug delivery, resulting in an obstacle for PDAC therapy. Considering the special tumor microenvironment of PDAC, we constructed a novel nanoparticle which is responsive to the membrane biomarker FAP-α on CAFs and near-infrared (NIR) laser irradiation. Small sized albumin nanoparticle of paclitaxel (HSA-PTX) with strong tumor-penetration ability was encapsulated into the CAP-(a FAP-α responsive cleavable amphiphilic peptide) modified thermosensitive liposomes (CAP-TSL). Moreover, IR-780, a photothermal agent, was incorporated into CAP-TSL to afford CAP-ITSL. The designed HSA-PTX@CAP-ITSL increased the drug retention of HSA-PTX in solid tumor and HSA-PTX was released via FAP-α (specifically expresses on CAFs) triggered. Under sequential stimulation of NIR laser irradiation, IR-780 produced hyperthermia to kill tumor cells and expand the tumor interstitial space at the same time, which further promoted the release of small sized HSA-PTX in deep tumor regions. Consequently, the excellent antitumor efficacy of HSA-PTX@CAP-ITSL was demonstrated in Pan 02 subcutaneous and orthotopic tumor mouse models. Therefore, HSA-PTX@CAP-ITSL well combined chemotherapy with photothermal therapy, providing a promising drug delivery strategy for PDAC treatment.
中文翻译:

通过双重反应脂质-白蛋白纳米颗粒靶向癌症相关的成纤维细胞,以增强胰腺肿瘤治疗的药物灌注。
胰腺导管腺癌(PDAC)富含癌症相关的成纤维细胞(CAF),它们参与肿瘤基质的形成。然而,PDAC的致密肿瘤基质对药物递送提出了主要障碍,从而导致了PDAC治疗的障碍。考虑到PDAC的特殊肿瘤微环境,我们构建了一种新型纳米颗粒,该纳米颗粒对CAF和近红外(NIR)激光辐照下的膜生物标记FAP-α有反应。紫杉醇(HSA-PTX)的小尺寸白蛋白纳米颗粒具有很强的肿瘤穿透能力,被包裹在CAP-(FAP-α反应性可裂解两亲性肽)修饰的热敏脂质体(CAP-TSL)中。而且,将光热剂IR-780掺入CAP-TSL中以提供CAP-ITSL。设计的HSA-PTX @ CAP-ITSL增加了HSA-PTX在实体瘤中的药物保留,并且通过触发FAP-α(在CAF上特异性表达)释放了HSA-PTX。在近红外激光的顺序刺激下,IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。
更新日期:2020-02-27
中文翻译:

通过双重反应脂质-白蛋白纳米颗粒靶向癌症相关的成纤维细胞,以增强胰腺肿瘤治疗的药物灌注。
胰腺导管腺癌(PDAC)富含癌症相关的成纤维细胞(CAF),它们参与肿瘤基质的形成。然而,PDAC的致密肿瘤基质对药物递送提出了主要障碍,从而导致了PDAC治疗的障碍。考虑到PDAC的特殊肿瘤微环境,我们构建了一种新型纳米颗粒,该纳米颗粒对CAF和近红外(NIR)激光辐照下的膜生物标记FAP-α有反应。紫杉醇(HSA-PTX)的小尺寸白蛋白纳米颗粒具有很强的肿瘤穿透能力,被包裹在CAP-(FAP-α反应性可裂解两亲性肽)修饰的热敏脂质体(CAP-TSL)中。而且,将光热剂IR-780掺入CAP-TSL中以提供CAP-ITSL。设计的HSA-PTX @ CAP-ITSL增加了HSA-PTX在实体瘤中的药物保留,并且通过触发FAP-α(在CAF上特异性表达)释放了HSA-PTX。在近红外激光的顺序刺激下,IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。IR-780产生高热,杀死肿瘤细胞并同时扩大肿瘤间隙,进一步促进了小分子HSA-PTX在深部肿瘤区域的释放。因此,在Pan 02皮下和原位肿瘤小鼠模型中证明了HSA-PTX @ CAP-ITSL的优异抗肿瘤功效。因此,HSA-PTX @ CAP-ITSL很好地将化学疗法与光热疗法相结合,为PDAC治疗提供了有希望的药物递送策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号