当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Amphiphilic Polymer-Supported Strategy Enables Chemical Transformations under Anhydrous Conditions for DNA-Encoded Library Synthesis.
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-02-10 , DOI: 10.1021/acscombsci.9b00164 Yves Ruff 1 , Roberto Martinez 1 , Xavier Pellé 1 , Pierre Nimsgern 1 , Pascale Fille 1 , Maxim Ratnikov 2 , Frédéric Berst 1
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-02-10 , DOI: 10.1021/acscombsci.9b00164 Yves Ruff 1 , Roberto Martinez 1 , Xavier Pellé 1 , Pierre Nimsgern 1 , Pascale Fille 1 , Maxim Ratnikov 2 , Frédéric Berst 1
Affiliation
|
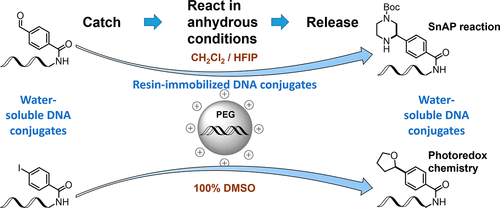
The use of DNA-encoded libraries has emerged as a powerful hit generation technology. Combining the power of combinatorial chemistry to enumerate large compound collections with the efficiency of affinity selection in pools, the methodology makes it possible to interrogate vast chemical space against biological targets of pharmaceutical relevance. Thus, the chemical transformations employed for the synthesis of encoded libraries play a crucial role in the identification of diverse and drug-like starting points. Currently established transformations have mostly been limited to water-compatible reactions to accommodate the growing oligonucleotide tag. Herein, we describe the development of a practical catch-and-release methodology utilizing a cationic, amphiphilic PEG-based polymer to perform chemical transformations on immobilized DNA conjugates under anhydrous conditions. We demonstrate the usefulness of our APTAC (amphiphilic polymer-facilitated transformations under anhydrous conditions) approach by performing several challenging transformations on DNA-conjugated small molecules in pure organic solvents: the addition of a carbanion equivalent to a DNA-conjugated ketone in tetrahydrofuran, the synthesis of saturated heterocycles using the tin (Sn) amine protocol (SnAP) in dichloromethane, and the dual-catalytic (Ir/Ni) metallaphotoredox decarboxylative cross-coupling of carboxylic acids to DNA-conjugated aryl halides in DMSO. In addition, we demonstrate the feasibility of the latter in multititer-plate format.
中文翻译:

两亲聚合物支持的策略可以在无水条件下进行化学转化,以进行DNA编码的文库合成。
DNA编码文库的使用已成为一种强大的命中生成技术。该方法结合了组合化学的功能(可枚举大量化合物)和池中亲和力选择的效率,使该方法有可能针对药物相关的生物学目标询问广阔的化学空间。因此,用于合成编码文库的化学转化在鉴定各种和类似药物的起点中起着至关重要的作用。当前建立的转化主要限于与水相容的反应,以适应不断增长的寡核苷酸标签。在此,我们描述了一种利用阳离子的实际捕集和释放方法的发展,两性基于PEG的聚合物,可在无水条件下对固定的DNA偶联物进行化学转化。我们通过在纯有机溶剂中对DNA共轭小分子进行一些具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属-金属氧化还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。我们通过在纯有机溶剂中对DNA共轭小分子进行几次具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及在DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属双氧还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。我们通过在纯有机溶剂中对DNA共轭小分子进行几次具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及在DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属双氧还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。在四氢呋喃中添加与DNA共轭的酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环以及双催化(Ir / Ni)金属氧还原氧化脱羧交叉偶联羧酸与DMSO中与DNA共轭的芳基卤化物。另外,我们证明了后者在多滴定板形式中的可行性。在四氢呋喃中添加与DNA共轭的酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环以及双催化(Ir / Ni)金属氧还原氧化脱羧交叉偶联羧酸与DMSO中与DNA共轭的芳基卤化物。另外,我们证明了后者在多滴定板形式中的可行性。
更新日期:2020-02-27
中文翻译:

两亲聚合物支持的策略可以在无水条件下进行化学转化,以进行DNA编码的文库合成。
DNA编码文库的使用已成为一种强大的命中生成技术。该方法结合了组合化学的功能(可枚举大量化合物)和池中亲和力选择的效率,使该方法有可能针对药物相关的生物学目标询问广阔的化学空间。因此,用于合成编码文库的化学转化在鉴定各种和类似药物的起点中起着至关重要的作用。当前建立的转化主要限于与水相容的反应,以适应不断增长的寡核苷酸标签。在此,我们描述了一种利用阳离子的实际捕集和释放方法的发展,两性基于PEG的聚合物,可在无水条件下对固定的DNA偶联物进行化学转化。我们通过在纯有机溶剂中对DNA共轭小分子进行一些具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属-金属氧化还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。我们通过在纯有机溶剂中对DNA共轭小分子进行几次具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及在DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属双氧还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。我们通过在纯有机溶剂中对DNA共轭小分子进行几次具有挑战性的转化,证明了APTAC(无水条件下两亲聚合物促进的转化)方法的有用性:在四氢呋喃中添加与DNA共轭酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环,以及在DMSO中羧酸与DNA共轭芳基卤化物的双催化(Ir / Ni)金属双氧还原脱羧交叉偶联。另外,我们证明了后者在多滴定板形式中的可行性。在四氢呋喃中添加与DNA共轭的酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环以及双催化(Ir / Ni)金属氧还原氧化脱羧交叉偶联羧酸与DMSO中与DNA共轭的芳基卤化物。另外,我们证明了后者在多滴定板形式中的可行性。在四氢呋喃中添加与DNA共轭的酮等效的碳负离子,在二氯甲烷中使用锡(Sn)胺规程(SnAP)合成饱和杂环以及双催化(Ir / Ni)金属氧还原氧化脱羧交叉偶联羧酸与DMSO中与DNA共轭的芳基卤化物。另外,我们证明了后者在多滴定板形式中的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号