当前位置:
X-MOL 学术
›
Insect Biochem. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
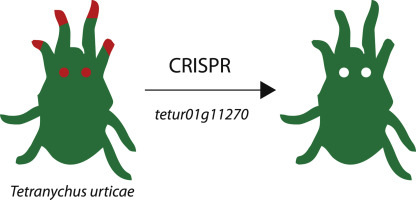
Targeted mutagenesis using CRISPR-Cas9 in the chelicerate herbivore Tetranychus urticae.
Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.ibmb.2020.103347 Wannes Dermauw 1 , Wim Jonckheere 1 , Maria Riga 2 , Ioannis Livadaras 2 , John Vontas 3 , Thomas Van Leeuwen 1
Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.ibmb.2020.103347 Wannes Dermauw 1 , Wim Jonckheere 1 , Maria Riga 2 , Ioannis Livadaras 2 , John Vontas 3 , Thomas Van Leeuwen 1
Affiliation
|
The use of CRISPR-Cas9 has revolutionized functional genetic work in many organisms, including more and more insect species. However, successful gene editing or genetic transformation has not yet been reported for chelicerates, the second largest group of terrestrial animals. Within this group, some mite and tick species are economically very important for agriculture and human health, and the availability of a gene-editing tool would be a significant advancement for the field. Here, we report on the use of CRISPR-Cas9 in the spider mite Tetranychus urticae. The ovary of virgin adult females was injected with a mix of Cas9 and sgRNAs targeting the phytoene desaturase gene. Natural mutants of this laterally transferred gene have previously shown an easy-to-score albino phenotype. Albino sons of injected virgin females were mated with wild-type females, and two independent transformed lines where created and further characterized. Albinism inherited as a recessive monogenic trait. Sequencing of the complete target-gene of both lines revealed two different lesions at expected locations near the PAM site in the target-gene. Both lines did not genetically complement each other in dedicated crosses, nor when crossed to a reference albino strain with a known genetic defect in the same gene. In conclusion, two independent mutagenesis events were induced in the spider mite T. urticae using CRISPR-Cas9, hereby providing proof-of-concept that CRISPR-Cas9 can be used to create gene knockouts in mites.
中文翻译:

使用CRISPR-Cas9在螯状草食性四叶虫(Tetranychus urticae)中进行定向诱变。
CRISPR-Cas9的使用彻底改变了许多生物体中的功能遗传工作,包括越来越多的昆虫。但是,尚未有关于螯虫的成功基因编辑或遗传转化的报道,螯虫是第二大陆生动物。在这个群体中,某些螨虫和壁虱物种在经济上对农业和人类健康非常重要,而基因编辑工具的可用性将对该领域产生重大的进步。在这里,我们报道了CRISPR-Cas9在红蜘蛛Tetranychus urticae中的使用。给未成年雌性卵巢注射Cas9和靶向八氢番茄红素去饱和酶基因的sgRNA的混合物。该横向转移的基因的天然突变体以前已经显示出易于得分的白化病表型。注射的处女雌性的白化儿子与野生型雌性交配,并创建了两个独立的转化系并对其进行了进一步表征。白化病是隐性的单基因特征。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在红蜘蛛螨中诱导了两个独立的诱变事件,从而提供了概念证明CRISPR-Cas9可用于在螨中创建基因敲除。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在蜘蛛红斑狼疮中诱导了两个独立的诱变事件,从而提供了CRISPR-Cas9可用于在螨中创建基因敲除的概念证明。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在蜘蛛红斑狼疮中诱导了两个独立的诱变事件,从而提供了CRISPR-Cas9可用于在螨中创建基因敲除的概念证明。
更新日期:2020-02-27
中文翻译:

使用CRISPR-Cas9在螯状草食性四叶虫(Tetranychus urticae)中进行定向诱变。
CRISPR-Cas9的使用彻底改变了许多生物体中的功能遗传工作,包括越来越多的昆虫。但是,尚未有关于螯虫的成功基因编辑或遗传转化的报道,螯虫是第二大陆生动物。在这个群体中,某些螨虫和壁虱物种在经济上对农业和人类健康非常重要,而基因编辑工具的可用性将对该领域产生重大的进步。在这里,我们报道了CRISPR-Cas9在红蜘蛛Tetranychus urticae中的使用。给未成年雌性卵巢注射Cas9和靶向八氢番茄红素去饱和酶基因的sgRNA的混合物。该横向转移的基因的天然突变体以前已经显示出易于得分的白化病表型。注射的处女雌性的白化儿子与野生型雌性交配,并创建了两个独立的转化系并对其进行了进一步表征。白化病是隐性的单基因特征。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在红蜘蛛螨中诱导了两个独立的诱变事件,从而提供了概念证明CRISPR-Cas9可用于在螨中创建基因敲除。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在蜘蛛红斑狼疮中诱导了两个独立的诱变事件,从而提供了CRISPR-Cas9可用于在螨中创建基因敲除的概念证明。两条品系的完整靶基因测序表明,在靶基因中PAM位点附近的预期位置有两个不同的病变。两条系在专门的杂交中都没有遗传互补,也没有与同一基因中已知遗传缺陷的参照白化菌株杂交。总之,使用CRISPR-Cas9在蜘蛛红斑狼疮中诱导了两个独立的诱变事件,从而提供了CRISPR-Cas9可用于在螨中创建基因敲除的概念证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号