当前位置:
X-MOL 学术
›
Genet. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG.
Genetics in Medicine ( IF 8.8 ) Pub Date : 2020-02-27 , DOI: 10.1038/s41436-020-0767-8 Peter Witters 1, 2 , Shawn Tahata 3 , Rita Barone 4 , Katrin Õunap 5, 6 , Ramona Salvarinova 7 , Sabine Grønborg 8 , George Hoganson 9 , Fernando Scaglia 10, 11, 12 , Andrea Margaret Lewis 10 , Mari Mori 13 , Jolanta Sykut-Cegielska 14 , Andrew Edmondson 15 , Miao He 16 , Eva Morava 1, 2, 3
Genetics in Medicine ( IF 8.8 ) Pub Date : 2020-02-27 , DOI: 10.1038/s41436-020-0767-8 Peter Witters 1, 2 , Shawn Tahata 3 , Rita Barone 4 , Katrin Õunap 5, 6 , Ramona Salvarinova 7 , Sabine Grønborg 8 , George Hoganson 9 , Fernando Scaglia 10, 11, 12 , Andrea Margaret Lewis 10 , Mari Mori 13 , Jolanta Sykut-Cegielska 14 , Andrew Edmondson 15 , Miao He 16 , Eva Morava 1, 2, 3
Affiliation
|
PURPOSE
We studied galactose supplementation in SLC35A2-congenital disorder of glycosylation (SLC35A2-CDG), caused by monoallelic pathogenic variants in SLC35A2 (Xp11.23), encoding the endoplasmic reticulum (ER) and Golgi UDP-galactose transporter. Patients present with epileptic encephalopathy, developmental disability, growth deficiency, and dysmorphism.
METHODS
Ten patients with SLC35A2-CDG were supplemented with oral D-galactose for 18 weeks in escalating doses up to 1.5 g/kg/day. Outcome was assessed using the Nijmegen Pediatric CDG Rating Scale (NPCRS, ten patients) and by glycomics (eight patients).
RESULTS
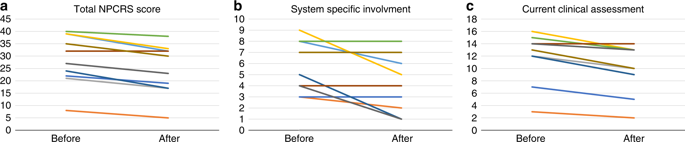
SLC35A2-CDG patients demonstrated improvements in overall Nijmegen Pediatric CDG Rating Scale (NPCRS) score (P = 0.008), the current clinical assessment (P = 0.007), and the system specific involvement (P = 0.042) domains. Improvements were primarily in growth and development with five patients resuming developmental progress, which included postural control, response to stimuli, and chewing and swallowing amelioration. Additionally, there were improvements in gastrointestinal symptoms and epilepsy. One patient in our study did not show any clinical improvement. Galactose supplementation improved patients' glycosylation with decreased ratios of incompletely formed to fully formed glycans (M-gal/disialo, P = 0.012 and monosialo/disialo, P = 0.017) and increased levels of a fully galactosylated N-glycan (P = 0.05).
CONCLUSIONS
Oral D-galactose supplementation results in clinical and biochemical improvement in SLC35A2-CDG. Galactose supplementation may partially overcome the Golgi UDP-galactose deficiency and improves galactosylation. Oral galactose is well tolerated and shows promise as dietary therapy.
中文翻译:

SLC35A2-CDG 中半乳糖补充剂的临床和生化改善。
目的我们研究了在 SLC35A2-先天性糖基化障碍 (SLC35A2-CDG) 中补充半乳糖,这是由 SLC35A2 (Xp11.23) 中的单等位基因致病变异引起的,编码内质网 (ER) 和高尔基体 UDP-半乳糖转运蛋白。患者表现为癫痫性脑病、发育障碍、生长缺陷和畸形。方法 10 名 SLC35A2-CDG 患者口服 D-半乳糖 18 周,剂量递增至 1.5 g/kg/天。使用奈梅亨儿科 CDG 评定量表(NPCRS,10 名患者)和糖组学(8 名患者)评估结果。结果 SLC35A2-CDG 患者在整体奈梅亨儿科 CDG 评定量表 (NPCRS) 评分 (P = 0.008)、当前临床评估 (P = 0.007) 和系统特定参与 (P = 0.042) 领域表现出改善。改善主要在生长和发育方面,有 5 名患者恢复发育进展,其中包括姿势控制、对刺激的反应以及咀嚼和吞咽的改善。此外,胃肠道症状和癫痫也有所改善。我们研究中的一名患者没有表现出任何临床改善。半乳糖补充剂改善了患者的糖基化,降低了未完全形成的聚糖与完全形成的聚糖的比例(M-gal/disialo,P = 0.012 和单唾液酸/disialo,P = 0.017),并增加了完全半乳糖基化的 N-聚糖的水平(P = 0.05) . 结论 口服 D-半乳糖补充剂导致 SLC35A2-CDG 的临床和生化改善。半乳糖补充剂可以部分克服高尔基体 UDP-半乳糖缺乏症并改善半乳糖基化。
更新日期:2020-02-27
中文翻译:

SLC35A2-CDG 中半乳糖补充剂的临床和生化改善。
目的我们研究了在 SLC35A2-先天性糖基化障碍 (SLC35A2-CDG) 中补充半乳糖,这是由 SLC35A2 (Xp11.23) 中的单等位基因致病变异引起的,编码内质网 (ER) 和高尔基体 UDP-半乳糖转运蛋白。患者表现为癫痫性脑病、发育障碍、生长缺陷和畸形。方法 10 名 SLC35A2-CDG 患者口服 D-半乳糖 18 周,剂量递增至 1.5 g/kg/天。使用奈梅亨儿科 CDG 评定量表(NPCRS,10 名患者)和糖组学(8 名患者)评估结果。结果 SLC35A2-CDG 患者在整体奈梅亨儿科 CDG 评定量表 (NPCRS) 评分 (P = 0.008)、当前临床评估 (P = 0.007) 和系统特定参与 (P = 0.042) 领域表现出改善。改善主要在生长和发育方面,有 5 名患者恢复发育进展,其中包括姿势控制、对刺激的反应以及咀嚼和吞咽的改善。此外,胃肠道症状和癫痫也有所改善。我们研究中的一名患者没有表现出任何临床改善。半乳糖补充剂改善了患者的糖基化,降低了未完全形成的聚糖与完全形成的聚糖的比例(M-gal/disialo,P = 0.012 和单唾液酸/disialo,P = 0.017),并增加了完全半乳糖基化的 N-聚糖的水平(P = 0.05) . 结论 口服 D-半乳糖补充剂导致 SLC35A2-CDG 的临床和生化改善。半乳糖补充剂可以部分克服高尔基体 UDP-半乳糖缺乏症并改善半乳糖基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号