Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Vivo Assembly of Nanoparticles Achieved through Synergy of Structure-Based Protein Engineering and Synthetic DNA Generates Enhanced Adaptive Immunity.
Advanced Science ( IF 15.1 ) Pub Date : 2020-02-27 , DOI: 10.1002/advs.201902802 Ziyang Xu 1, 2 , Megan C Wise 3 , Neethu Chokkalingam 1 , Susanne Walker 1 , Edgar Tello-Ruiz 1 , Sarah T C Elliott 1 , Alfredo Perales-Puchalt 1 , Peng Xiao 1 , Xizhou Zhu 1 , Ruth A Pumroy 2 , Paul D Fisher 3 , Katherine Schultheis 3 , Eric Schade 3 , Sergey Menis 4, 5, 6 , Stacy Guzman 1 , Hanne Andersen 7 , Kate E Broderick 3 , Laurent M Humeau 3 , Kar Muthumani 1 , Vera Moiseenkova-Bell 2 , William R Schief 4, 5, 6, 8 , David B Weiner 1 , Daniel W Kulp 1, 9
Advanced Science ( IF 15.1 ) Pub Date : 2020-02-27 , DOI: 10.1002/advs.201902802 Ziyang Xu 1, 2 , Megan C Wise 3 , Neethu Chokkalingam 1 , Susanne Walker 1 , Edgar Tello-Ruiz 1 , Sarah T C Elliott 1 , Alfredo Perales-Puchalt 1 , Peng Xiao 1 , Xizhou Zhu 1 , Ruth A Pumroy 2 , Paul D Fisher 3 , Katherine Schultheis 3 , Eric Schade 3 , Sergey Menis 4, 5, 6 , Stacy Guzman 1 , Hanne Andersen 7 , Kate E Broderick 3 , Laurent M Humeau 3 , Kar Muthumani 1 , Vera Moiseenkova-Bell 2 , William R Schief 4, 5, 6, 8 , David B Weiner 1 , Daniel W Kulp 1, 9
Affiliation
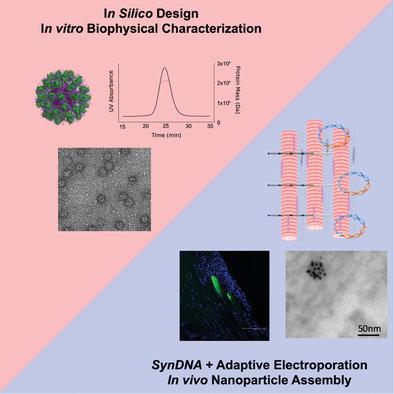
|
Nanotechnologies are considered to be of growing importance to the vaccine field. Through decoration of immunogens on multivalent nanoparticles, designed nanovaccines can elicit improved humoral immunity. However, significant practical and monetary challenges in large-scale production of nanovaccines have impeded their widespread clinical translation. Here, an alternative approach is illustrated integrating computational protein modeling and adaptive electroporation-mediated synthetic DNA delivery, thus enabling direct in vivo production of nanovaccines. DNA-launched nanoparticles are demonstrated displaying an HIV immunogen spontaneously self-assembled in vivo. DNA-launched nanovaccines induce stronger humoral responses than their monomeric counterparts in both mice and guinea pigs, and uniquely elicit CD8+ effector T-cell immunity as compared to recombinant protein nanovaccines. Improvements in vaccine responses recapitulate when DNA-launched nanovaccines with alternative scaffolds and decorated antigen are designed and evaluated. Finally, evaluation of functional immune responses induced by DLnanovaccines demonstrates that, in comparison to control mice or mice immunized with DNA-encoded hemagglutinin monomer, mice immunized with a DNA-launched hemagglutinin nanoparticle vaccine fully survive a lethal influenza challenge, and have substantially lower viral load, weight loss, and influenza-induced lung pathology. Additional study of these next-generation in vivo-produced nanovaccines may offer advantages for immunization against multiple disease targets.
中文翻译:

通过基于结构的蛋白质工程协同作用实现的纳米粒子体内组装,合成的DNA产生增强的适应性免疫力。
纳米技术被认为对疫苗领域越来越重要。通过在多价纳米颗粒上修饰免疫原,设计的纳米疫苗可以引发更高的体液免疫力。然而,纳米疫苗大规模生产中的重大实践和金钱挑战阻碍了其广泛的临床翻译。在这里,说明了一种替代方法,该方法整合了计算蛋白质建模和自适应电穿孔介导的合成DNA传递,从而可以在体内直接生产纳米疫苗。证明了DNA发射的纳米颗粒在体内自发地自我展示了HIV免疫原。在小鼠和豚鼠中,DNA发射的纳米疫苗比其单体对应物诱导的体液反应更强,与重组蛋白纳米疫苗相比,它具有独特的CD8 +效应T细胞免疫力。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或以DNA编码的血凝素单体免疫的小鼠相比,以DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒载量大大降低,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或以DNA编码的血凝素单体免疫的小鼠相比,以DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒载量大大降低,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。
更新日期:2020-04-21
中文翻译:

通过基于结构的蛋白质工程协同作用实现的纳米粒子体内组装,合成的DNA产生增强的适应性免疫力。
纳米技术被认为对疫苗领域越来越重要。通过在多价纳米颗粒上修饰免疫原,设计的纳米疫苗可以引发更高的体液免疫力。然而,纳米疫苗大规模生产中的重大实践和金钱挑战阻碍了其广泛的临床翻译。在这里,说明了一种替代方法,该方法整合了计算蛋白质建模和自适应电穿孔介导的合成DNA传递,从而可以在体内直接生产纳米疫苗。证明了DNA发射的纳米颗粒在体内自发地自我展示了HIV免疫原。在小鼠和豚鼠中,DNA发射的纳米疫苗比其单体对应物诱导的体液反应更强,与重组蛋白纳米疫苗相比,它具有独特的CD8 +效应T细胞免疫力。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。当设计和评估具有替代支架和修饰抗原的DNA发射的纳米疫苗时,疫苗反应的改善得以概括。最后,对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或用DNA编码的血凝素单体免疫的小鼠相比,用DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒水平要低得多负荷,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或以DNA编码的血凝素单体免疫的小鼠相比,以DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒载量大大降低,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。对DLnanovaccines诱导的功能性免疫应答的评估表明,与对照小鼠或以DNA编码的血凝素单体免疫的小鼠相比,以DNA发射的血凝素纳米颗粒疫苗免疫的小鼠可以完全抵抗致命的流感病毒攻击,并且病毒载量大大降低,体重减轻和流感引起的肺部病理。这些下一代体内产生的纳米疫苗的进一步研究可能为针对多种疾病靶标的免疫接种提供优势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号