当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
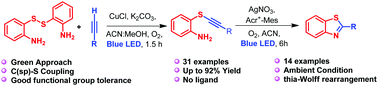
Visible light initiated amino group ortho-directed copper(i)-catalysed aerobic oxidative C(sp)-S coupling reaction: synthesis of substituted 2-phenylbenzothiazoles via thia-Wolff rearrangement.
Chemical Communications ( IF 4.9 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0cc00815j Mandapati Bhargava Reddy 1 , Ramasamy Anandhan 1
Chemical Communications ( IF 4.9 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0cc00815j Mandapati Bhargava Reddy 1 , Ramasamy Anandhan 1
Affiliation
|
A facile amino group ortho-directed visible-light-driven copper-catalysed aerobic oxidative C(sp)-S coupling reaction of a dimer of 2-aminothiophenol with terminal alkynes was achieved. This photochemical reaction shows an excellent conversion and chemoselectivity towards the formation of C(sp)-S coupling and has been employed for a wide range of thiol dimers, and alkynes. Furthermore, the synthetic utility of the synthesized alkynyl sulfides was demonstrated as a direct method for the construction of 2-phenylbenzothiazoles from the corresponding alkynyl sulfides via "thia-Wolff rearrangement" using AgNO3 and visible light using 9-mesityl-10-methylacridinium ions (Acr+-Mes) as photoredox catalyst system.
中文翻译:

可见光引发的氨基邻位定向铜(i)催化的好氧氧化C(sp)-S偶联反应:通过thia-Wolff重排合成取代的2-苯基苯并噻唑。
实现了2-氨基硫酚二聚体与末端炔烃的简单的氨基邻位定向可见光驱动的铜催化的需氧氧化C(sp)-S偶联反应。这种光化学反应显示出极好的转化率和对C(sp)-S偶联形成的化学选择性,已被广泛用于硫醇二聚体和炔烃。此外,合成的炔基硫醚的合成效用已证明是直接的方法,可通过相应的炔基硫醚使用AgNO3的“噻吩-沃尔夫重排”法和使用9-间苯甲基-10甲基ac啶离子的可见光从相应的炔基硫醚构建2-苯基苯并噻唑( Acr + -Mes)作为光氧化还原催化剂体系。
更新日期:2020-03-31
中文翻译:

可见光引发的氨基邻位定向铜(i)催化的好氧氧化C(sp)-S偶联反应:通过thia-Wolff重排合成取代的2-苯基苯并噻唑。
实现了2-氨基硫酚二聚体与末端炔烃的简单的氨基邻位定向可见光驱动的铜催化的需氧氧化C(sp)-S偶联反应。这种光化学反应显示出极好的转化率和对C(sp)-S偶联形成的化学选择性,已被广泛用于硫醇二聚体和炔烃。此外,合成的炔基硫醚的合成效用已证明是直接的方法,可通过相应的炔基硫醚使用AgNO3的“噻吩-沃尔夫重排”法和使用9-间苯甲基-10甲基ac啶离子的可见光从相应的炔基硫醚构建2-苯基苯并噻唑( Acr + -Mes)作为光氧化还原催化剂体系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号