当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High Stabilization of Immobilized Rhizomucor miehei Lipase by Additional Coating with Hydrophilic Crosslinked Polymers: Poly-allylamine/Aldehyde–dextran
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.procbio.2020.02.026 Janaina Cejudo-Sanches , Alejandro H. Orrego , Adriana Jaime-Mendoza , Rohollah Ghobadi , Sonia Moreno-Perez , Gloria Fernandez-Lorente , Javier Rocha-Martin , José M. Guisan
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.procbio.2020.02.026 Janaina Cejudo-Sanches , Alejandro H. Orrego , Adriana Jaime-Mendoza , Rohollah Ghobadi , Sonia Moreno-Perez , Gloria Fernandez-Lorente , Javier Rocha-Martin , José M. Guisan
|
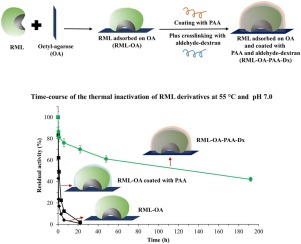
Abstract Immobilized enzymes have a very large surface region which is not in contact with the support surface and, thus, have potential as a target for novel stabilization strategies. In this paper, coating the surfaces of such enzymes with a highly hydrophilic and compact cross-linked poly-aminated polymer as a strategy to increase the thermal stability of the immobilized enzymes is proposed. In particular, Rhizomucor miehei lipase (RML) was immobilized by interfacial adsorption onto octyl-agarose and further coated with poly-allylamine (PAA), a polymer that is very rich in primary amino groups. Cross-linking of the PAA layer to coat the immobilized enzyme was carried out, in situ, by reaction with freshly oxidized dextran (aldehyde–dextran). The PAA layer only exerted moderate stabilizing effects (around 4-fold), but further cross-linking with aldehyde–dextran highly increased the stabilizing effects; the new derivative was 440-fold more stable than uncoated derivative at 55 °C and pH 7 and exhibited 6-fold more catalytic activity compared to the soluble enzyme used for immobilization. We hypothesize that the hydrophilicity of PAA reduces the exposure of internal hydrophobic pockets to the enzyme surface at high temperatures. Besides, the compactness of the polymer may reduce distortion of the enzyme surface during inactivation.
中文翻译:

通过额外包覆亲水性交联聚合物:聚烯丙胺/醛-葡聚糖,对固定化的米黑根霉脂肪酶具有高稳定性
摘要 固定化酶具有非常大的表面区域,不与载体表面接触,因此具有作为新型稳定策略靶点的潜力。在本文中,提出了用高度亲水且致密的交联多胺化聚合物涂覆此类酶的表面作为提高固定化酶热稳定性的策略。特别是,Rhizomucor miehei 脂肪酶 (RML) 通过界面吸附固定在辛基琼脂糖上,并进一步涂上聚烯丙胺 (PAA),这是一种富含伯氨基的聚合物。通过与新鲜氧化的葡聚糖(醛-葡聚糖)反应,原位进行 PAA 层的交联以覆盖固定化酶。PAA 层仅发挥适度的稳定作用(约 4 倍),但是与醛-葡聚糖进一步交联会大大增加稳定效果;在 55 °C 和 pH 7 条件下,新衍生物的稳定性比未包被的衍生物稳定 440 倍,与用于固定的可溶性酶相比,其催化活性高 6 倍。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。
更新日期:2020-05-01
中文翻译:

通过额外包覆亲水性交联聚合物:聚烯丙胺/醛-葡聚糖,对固定化的米黑根霉脂肪酶具有高稳定性
摘要 固定化酶具有非常大的表面区域,不与载体表面接触,因此具有作为新型稳定策略靶点的潜力。在本文中,提出了用高度亲水且致密的交联多胺化聚合物涂覆此类酶的表面作为提高固定化酶热稳定性的策略。特别是,Rhizomucor miehei 脂肪酶 (RML) 通过界面吸附固定在辛基琼脂糖上,并进一步涂上聚烯丙胺 (PAA),这是一种富含伯氨基的聚合物。通过与新鲜氧化的葡聚糖(醛-葡聚糖)反应,原位进行 PAA 层的交联以覆盖固定化酶。PAA 层仅发挥适度的稳定作用(约 4 倍),但是与醛-葡聚糖进一步交联会大大增加稳定效果;在 55 °C 和 pH 7 条件下,新衍生物的稳定性比未包被的衍生物稳定 440 倍,与用于固定的可溶性酶相比,其催化活性高 6 倍。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。我们假设 PAA 的亲水性减少了内部疏水口袋在高温下对酶表面的暴露。此外,聚合物的致密性可以减少失活过程中酶表面的变形。


























 京公网安备 11010802027423号
京公网安备 11010802027423号