当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of new benzothiazole derivatives bearing thiadiazole as monoamine oxidase inhibitors
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-02-25 , DOI: 10.1002/jhet.3942 Ulviye Acar Çevik 1, 2 , Derya Osmaniye 1, 2 , Begüm N. Sağlik 1, 2 , Serkan Levent 1, 2 , Betül K. Çavuşoğlu 1, 2 , Abdullah B. Karaduman 3 , Ümide D. Özkay 4 , Yusuf Özkay 1, 2 , Zafer A. Kaplancikli 1 , Gülhan Turan 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-02-25 , DOI: 10.1002/jhet.3942 Ulviye Acar Çevik 1, 2 , Derya Osmaniye 1, 2 , Begüm N. Sağlik 1, 2 , Serkan Levent 1, 2 , Betül K. Çavuşoğlu 1, 2 , Abdullah B. Karaduman 3 , Ümide D. Özkay 4 , Yusuf Özkay 1, 2 , Zafer A. Kaplancikli 1 , Gülhan Turan 1
Affiliation
|
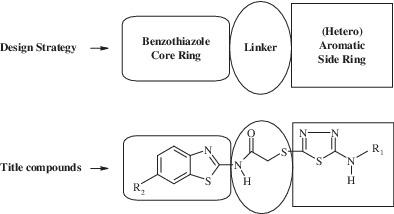
Monoamine oxidases (MAO) are enzymes that catalyze the oxidative deamination of monoamines such as dopamine, noradrenaline, adrenaline, and serotonin. Recent studies have shown that numerous benzothiazole derivatives exhibit hMAO inhibitory activity in the micromolar concentration range. In this study, a novel series of benzothiazole‐thiadiazole (5a‐5l) was synthesized and characterized their chemical structures by 1H‐NMR, 13C‐NMR, and Mass spectroscopy. These compounds were evaluated as inhibitors for types A and B MAO enzymes. Compounds 5f and 5l were the most active derivatives in the series with an IC50 values of 0.107 ± 0.003 and 0.128 ± 0.004, respectively. Furthermore, cytotoxicity of compounds 5f and 5l were investigated and found as non‐cytotoxic.
中文翻译:

含噻二唑作为单胺氧化酶抑制剂的新型苯并噻唑衍生物的合成
单胺氧化酶(MAO)是催化单胺(如多巴胺,去甲肾上腺素,肾上腺素和血清素)氧化脱氨的酶。最近的研究表明,许多苯并噻唑衍生物在微摩尔浓度范围内均具有h MAO抑制活性。在这项研究中,合成了一系列新颖的苯并噻唑-噻二唑(5a-5l),并通过1 H-NMR,13 C-NMR和质谱表征了它们的化学结构。这些化合物被评估为A型和B型MAO酶的抑制剂。化合物5f和5l是具有IC 50的系列中活性最高的衍生物值分别为0.107±0.003和0.128±0.004。此外,对化合物5f和5l的细胞毒性进行了研究,发现为非细胞毒性。
更新日期:2020-02-25
中文翻译:

含噻二唑作为单胺氧化酶抑制剂的新型苯并噻唑衍生物的合成
单胺氧化酶(MAO)是催化单胺(如多巴胺,去甲肾上腺素,肾上腺素和血清素)氧化脱氨的酶。最近的研究表明,许多苯并噻唑衍生物在微摩尔浓度范围内均具有h MAO抑制活性。在这项研究中,合成了一系列新颖的苯并噻唑-噻二唑(5a-5l),并通过1 H-NMR,13 C-NMR和质谱表征了它们的化学结构。这些化合物被评估为A型和B型MAO酶的抑制剂。化合物5f和5l是具有IC 50的系列中活性最高的衍生物值分别为0.107±0.003和0.128±0.004。此外,对化合物5f和5l的细胞毒性进行了研究,发现为非细胞毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号