当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Purifying stem cell-derived red blood cells: a high-throughput label-free downstream processing strategy based on microfluidic spiral inertial separation and membrane filtration.
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-02-26 , DOI: 10.1002/bit.27319 Ewa Guzniczak 1 , Oliver Otto 2, 3 , Graeme Whyte 1 , Tamir Chandra 4 , Neil A Robertson 4 , Nik Willoughby 1 , Melanie Jimenez 5 , Helen Bridle 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-02-26 , DOI: 10.1002/bit.27319 Ewa Guzniczak 1 , Oliver Otto 2, 3 , Graeme Whyte 1 , Tamir Chandra 4 , Neil A Robertson 4 , Nik Willoughby 1 , Melanie Jimenez 5 , Helen Bridle 1
Affiliation
|
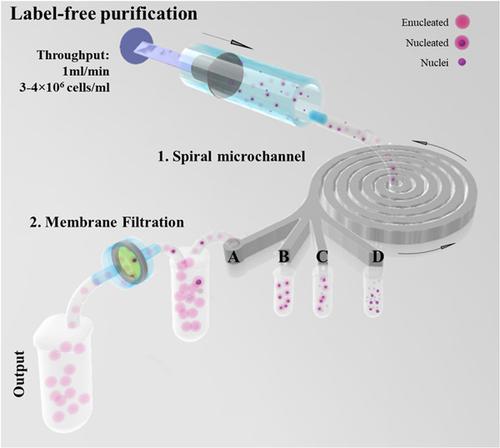
Cell‐based therapeutics, such as in vitro manufactured red blood cells (mRBCs), are different to traditional biopharmaceutical products (the final product being the cells themselves as opposed to biological molecules such as proteins) and that presents a challenge of developing new robust and economically feasible manufacturing processes, especially for sample purification. Current purification technologies have limited throughput, rely on expensive fluorescent or magnetic immunolabeling with a significant (up to 70%) cell loss and quality impairment. To address this challenge, previously characterized mechanical properties of umbilical cord blood CD34+ cells undergoing in vitro erythropoiesis were used to develop an mRBC purification strategy. The approach consists of two main stages: (a) a microfluidic separation using inertial focusing for deformability‐based sorting of enucleated cells (mRBC) from nuclei and nucleated cells resulting in 70% purity and (b) membrane filtration to enhance the purity to 99%. Herein, we propose a new route for high‐throughput (processing millions of cells/min and mls of medium/min) purification process for mRBC, leading to high mRBC purity while maintaining cell integrity and no alterations in their global gene expression profile. Further adaption of this separation approach offers a potential route for processing of a wide range of cellular products.
中文翻译:

纯化干细胞衍生的红细胞:一种基于微流体螺旋惯性分离和膜过滤的高通量无标记下游处理策略。
基于细胞的疗法,例如体外制造的红细胞 (mRBC),与传统的生物制药产品(最终产品是细胞本身,而不是蛋白质等生物分子)不同,这对开发新的健壮和经济可行的制造工艺,特别是对于样品纯化。当前的纯化技术具有有限的通量,依赖于昂贵的荧光或磁性免疫标记,具有显着(高达 70%)的细胞损失和质量损害。为了应对这一挑战,使用先前表征的体外红细胞生成的脐带血 CD34+ 细胞的机械特性来开发 mRBC 纯化策略。该方法包括两个主要阶段:(a) 使用惯性聚焦进行微流体分离,以基于变形能力从细胞核和有核细胞中分选去核细胞 (mRBC),纯度为 70%;(b) 膜过滤将纯度提高至 99%。在此,我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,从而在保持细胞完整性的同时保持高 mRBC 纯度并且不改变其整体基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,导致高 mRBC 纯度,同时保持细胞完整性并且不改变其全局基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,导致高 mRBC 纯度,同时保持细胞完整性并且不改变其全局基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。
更新日期:2020-02-26
中文翻译:

纯化干细胞衍生的红细胞:一种基于微流体螺旋惯性分离和膜过滤的高通量无标记下游处理策略。
基于细胞的疗法,例如体外制造的红细胞 (mRBC),与传统的生物制药产品(最终产品是细胞本身,而不是蛋白质等生物分子)不同,这对开发新的健壮和经济可行的制造工艺,特别是对于样品纯化。当前的纯化技术具有有限的通量,依赖于昂贵的荧光或磁性免疫标记,具有显着(高达 70%)的细胞损失和质量损害。为了应对这一挑战,使用先前表征的体外红细胞生成的脐带血 CD34+ 细胞的机械特性来开发 mRBC 纯化策略。该方法包括两个主要阶段:(a) 使用惯性聚焦进行微流体分离,以基于变形能力从细胞核和有核细胞中分选去核细胞 (mRBC),纯度为 70%;(b) 膜过滤将纯度提高至 99%。在此,我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,从而在保持细胞完整性的同时保持高 mRBC 纯度并且不改变其整体基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,导致高 mRBC 纯度,同时保持细胞完整性并且不改变其全局基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。我们提出了一种新的 mRBC 高通量(处理数百万个细胞/分钟和 mls 的培养基/分钟)纯化过程的新途径,导致高 mRBC 纯度,同时保持细胞完整性并且不改变其全局基因表达谱。这种分离方法的进一步调整为处理各种细胞产品提供了潜在的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号