当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibrium for the Reciprocal Quaternary System (Na+, Cs+//Cl–, SO42––H2O) at T = 298.15 K and 0.1 MPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jced.9b00783 Miaosen Shi 1 , Shangqing Chen 1 , Jiayin Hu 1 , Yafei Guo 1 , Tianlong Deng 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-25 , DOI: 10.1021/acs.jced.9b00783 Miaosen Shi 1 , Shangqing Chen 1 , Jiayin Hu 1 , Yafei Guo 1 , Tianlong Deng 1
Affiliation
|
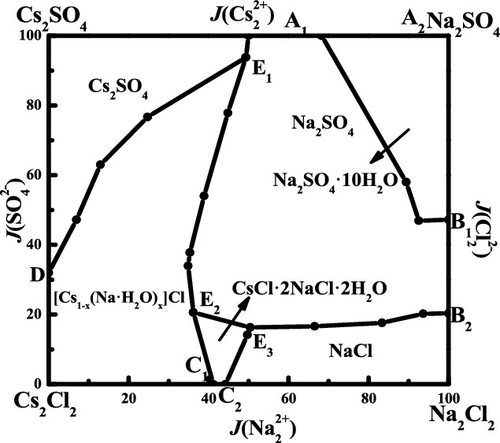
The phase equilibria and phase diagrams are very helpful in describing the geochemical behaviors of aqueous solution and provide great guidance for the separation of salts. In this paper, solid–liquid phase equilibrium for the reciprocal quaternary system (Na+, Cs+//Cl–, SO42––H2O) at T = 298.15 K and 0.1 MPa was carried out with an isothermal dissolution method. The solubilities and physicochemical properties (densities and refractive indices) in this reciprocal quaternary system (Na+, Cs+//Cl–, SO42––H2O) were investigated. The dry-salt and water-phase diagrams, as well as physicochemical properties versus composition diagrams were also established. Three invariant points, eight univariant curves, and six crystallization regions corresponding to cesium sulfate (Cs2SO4), halite (NaCl), thenardite (Na2SO4), mirabilite (Na2SO4·10H2O), a solid solution ([Cs1–x(Na·H2O)x]Cl), and a double salt (CsCl·2NaCl·2H2O) could be found in this system. The crystallization region of thenardite was the largest, indicating thenardite could be easily crystallized and separated from this system. In addition, the physicochemical properties showed a regular change with the increase of Jänecke index values of J(Na22+).
中文翻译:

互逆四元体系(Na +,Cs + // Cl –,SO 4 2 – –H 2 O)在T = 298.15 K和0.1 MPa时的固液平衡
相平衡和相图对于描述水溶液的地球化学行为非常有帮助,并为分离盐提供了很好的指导。本文采用等温溶解法,在T = 298.15 K和0.1 MPa的条件下,对四元体系(Na +,Cs + // Cl –,SO 4 2 – –H 2 O)进行固液平衡。。互易四元体系(Na +,Cs + // Cl –,SO 4 2– –H 2)的溶解度和理化性质(密度和折射率)O)被调查。还建立了干盐和水相图,以及理化性质与组成图。固态的硫酸铯(Cs 2 SO 4),盐石(NaCl),芒硝(Na 2 SO 4),芒硝(Na 2 SO 4 ·10H 2 O)的三个不变点,八个单变曲线和六个结晶区域溶液([Cs 1- x(Na·H 2 O)x ] Cl)和复盐(CsCl·2NaCl·2H 2O)可以在此系统中找到。芒硝的结晶区域最大,表明芒硝易于结晶并与该体系分离。此外,理化性质显示出随J(Na 2 2+)的Jänecke指数值的增加而有规律的变化。
更新日期:2020-02-25
中文翻译:

互逆四元体系(Na +,Cs + // Cl –,SO 4 2 – –H 2 O)在T = 298.15 K和0.1 MPa时的固液平衡
相平衡和相图对于描述水溶液的地球化学行为非常有帮助,并为分离盐提供了很好的指导。本文采用等温溶解法,在T = 298.15 K和0.1 MPa的条件下,对四元体系(Na +,Cs + // Cl –,SO 4 2 – –H 2 O)进行固液平衡。。互易四元体系(Na +,Cs + // Cl –,SO 4 2– –H 2)的溶解度和理化性质(密度和折射率)O)被调查。还建立了干盐和水相图,以及理化性质与组成图。固态的硫酸铯(Cs 2 SO 4),盐石(NaCl),芒硝(Na 2 SO 4),芒硝(Na 2 SO 4 ·10H 2 O)的三个不变点,八个单变曲线和六个结晶区域溶液([Cs 1- x(Na·H 2 O)x ] Cl)和复盐(CsCl·2NaCl·2H 2O)可以在此系统中找到。芒硝的结晶区域最大,表明芒硝易于结晶并与该体系分离。此外,理化性质显示出随J(Na 2 2+)的Jänecke指数值的增加而有规律的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号