当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polyacrylic acid based plasma fractionation for the production of albumin and IgG: Compatibility with existing commercial downstream processes.
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-01-12 , DOI: 10.1002/bit.27265 Karl B McCann 1 , James Van Alstine 2, 3 , Jose Martinez 1 , Jamil Shanagar 4 , Joseph Bertolini 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-01-12 , DOI: 10.1002/bit.27265 Karl B McCann 1 , James Van Alstine 2, 3 , Jose Martinez 1 , Jamil Shanagar 4 , Joseph Bertolini 1
Affiliation
|
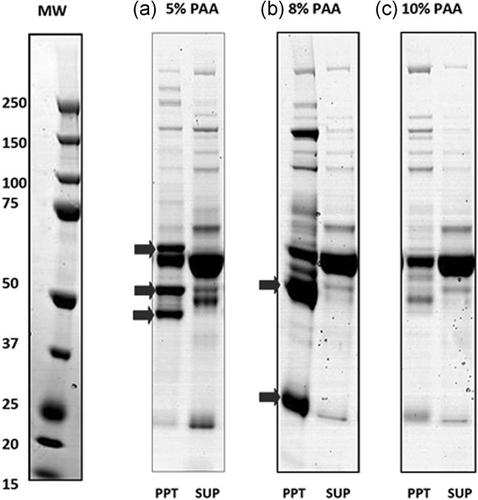
Commercial fractionation of human plasma into immunoglobulin- and albumin-rich fractions is often initiated with sequential cold ethanol-based precipitation methods, which have changed little over the past 70 years. The required low temperature (-4 to -8°C) and high concentrations of ethanol 8-40%) necessitate large-scale fixed processing lines, and major capital investment and operating costs. The resulting fractions are then further purified by ethanol based precipitation or chromatographic procedures to obtain the purified final product. Aqueous polyacrylic acid (PAA) based precipitation, which readily interfaces with existing downstream processing, could offer advantages with respect to cost, safety, environmental impact, and flexibility. Sequential precipitation with 7%, 12%, and 20% (w/v) solutions of PAA 8000 in the presence of a kosmotropic salt (sodium citrate) gave fibrinogen-, immunoglobulin-, and albumin-rich fractions with 80-90% yield and 64%, 55%, and 82% purity, respectively. Further purification of the IgG-rich precipitate by caprylic acid precipitation and anion exchange chromatography, achieved a target purity of >99%. This was also achieved for the downstream processing of the albumin-rich precipitate using a two-step ion exchange chromatographic procedure. This work shows that PAA precipitation can be used in place of cold ethanol precipitation to generate crude IgG and albumin fractions which can be purified to final products of acceptable purity.
中文翻译:

用于生产白蛋白和IgG的基于聚丙烯酸的血浆分馏:与现有商业下游工艺的兼容性。
通常将人血浆分为富含免疫球蛋白和白蛋白的部分进行商业分离,这是通过连续的基于乙醇的冷沉淀方法开始的,在过去的70年中,这种方法变化不大。所需的低温(-4至-8°C)和高浓度的乙醇(8-40%)需要大规模的固定生产线,并需要大量的资金投入和运营成本。然后将所得级分通过基于乙醇的沉淀或色谱法进一步纯化,以获得纯化的最终产物。聚丙烯酸(PAA)水溶液基沉淀物易于与现有下游工艺相接,可在成本,安全性,环境影响和灵活性方面提供优势。依次降水分别为7%,12%,和20%(w / v)的PAA 8000溶液在均溶盐(柠檬酸钠)的存在下,可得到富含纤维蛋白原,免疫球蛋白和白蛋白的级分,产率为80-90%,分别为64%,55%和82分别为%纯度。通过辛酸沉淀和阴离子交换色谱进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,这些馏分可以纯化为可接受纯度的最终产物。通过辛酸沉淀和阴离子交换色谱法进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以使用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,可以将其纯化为纯度可接受的最终产物。通过辛酸沉淀和阴离子交换色谱进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以使用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,可以将其纯化为纯度可接受的最终产物。
更新日期:2020-03-09
中文翻译:

用于生产白蛋白和IgG的基于聚丙烯酸的血浆分馏:与现有商业下游工艺的兼容性。
通常将人血浆分为富含免疫球蛋白和白蛋白的部分进行商业分离,这是通过连续的基于乙醇的冷沉淀方法开始的,在过去的70年中,这种方法变化不大。所需的低温(-4至-8°C)和高浓度的乙醇(8-40%)需要大规模的固定生产线,并需要大量的资金投入和运营成本。然后将所得级分通过基于乙醇的沉淀或色谱法进一步纯化,以获得纯化的最终产物。聚丙烯酸(PAA)水溶液基沉淀物易于与现有下游工艺相接,可在成本,安全性,环境影响和灵活性方面提供优势。依次降水分别为7%,12%,和20%(w / v)的PAA 8000溶液在均溶盐(柠檬酸钠)的存在下,可得到富含纤维蛋白原,免疫球蛋白和白蛋白的级分,产率为80-90%,分别为64%,55%和82分别为%纯度。通过辛酸沉淀和阴离子交换色谱进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,这些馏分可以纯化为可接受纯度的最终产物。通过辛酸沉淀和阴离子交换色谱法进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以使用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,可以将其纯化为纯度可接受的最终产物。通过辛酸沉淀和阴离子交换色谱进一步纯化富含IgG的沉淀,目标纯度> 99%。使用两步离子交换色谱法对富含白蛋白的沉淀物进行下游处理也可以实现这一点。这项工作表明,可以使用PAA沉淀代替冷乙醇沉淀来生成粗制IgG和白蛋白馏分,可以将其纯化为纯度可接受的最终产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号