当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Envelope stress responses defend against type six secretion system attacks independently of immunity proteins.
Nature Microbiology ( IF 28.3 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41564-020-0672-6 Steven J Hersch 1 , Nobuhiko Watanabe 2 , Maria Silvina Stietz 1 , Kevin Manera 1 , Fatima Kamal 1 , Brianne Burkinshaw 1 , Linh Lam 1 , Alexander Pun 1 , Meixin Li 1 , Alexei Savchenko 2 , Tao G Dong 1, 3
Nature Microbiology ( IF 28.3 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41564-020-0672-6 Steven J Hersch 1 , Nobuhiko Watanabe 2 , Maria Silvina Stietz 1 , Kevin Manera 1 , Fatima Kamal 1 , Brianne Burkinshaw 1 , Linh Lam 1 , Alexander Pun 1 , Meixin Li 1 , Alexei Savchenko 2 , Tao G Dong 1, 3
Affiliation
|
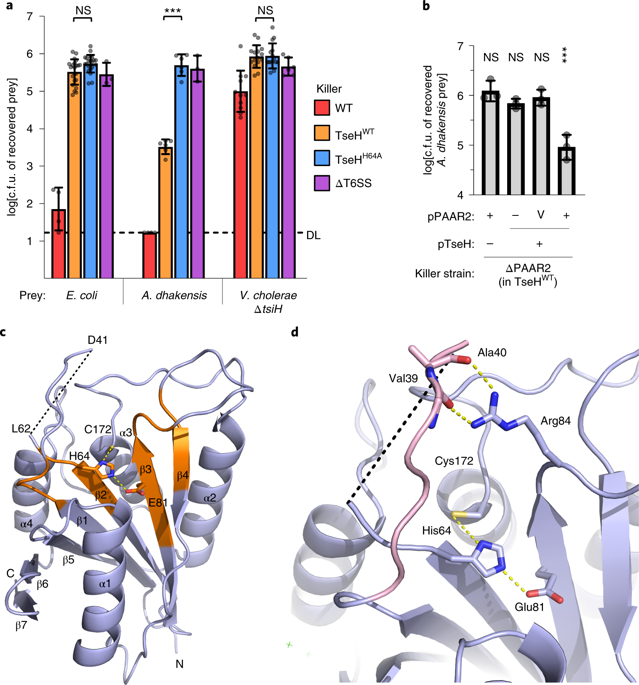
The arms race among microorganisms is a key driver in the evolution of not only the weapons but also defence mechanisms. Many Gram-negative bacteria use the type six secretion system (T6SS) to deliver toxic effectors directly into neighbouring cells. Defence against effectors requires cognate immunity proteins. However, here we show immunity-independent protection mediated by envelope stress responses in Escherichia coli and Vibrio cholerae against a V. cholerae T6SS effector, TseH. We demonstrate that TseH is a PAAR-dependent species-specific effector highly potent against Aeromonas species but not against its V. cholerae immunity mutant or E. coli. A structural analysis reveals TseH is probably a NlpC/P60-family cysteine endopeptidase. We determine that two envelope stress-response pathways, Rcs and BaeSR, protect E. coli from TseH toxicity by mechanisms including capsule synthesis. The two-component system WigKR (VxrAB) is critical for protecting V. cholerae from its own T6SS despite expressing immunity genes. WigR also regulates T6SS expression, suggesting a dual role in attack and defence. This deepens our understanding of how bacteria survive T6SS attacks and suggests that defence against the T6SS represents a major selective pressure driving the evolution of species-specific effectors and protective mechanisms mediated by envelope stress responses and capsule synthesis.
中文翻译:

包膜应激反应独立于免疫蛋白防御六型分泌系统的攻击。
微生物之间的军备竞赛不仅是武器发展的关键驱动力,也是防御机制发展的关键驱动力。许多革兰氏阴性细菌使用六型分泌系统(T6SS)将毒性效应子直接传递到邻近细胞中。对效应子的防御需要同源的免疫蛋白。但是,这里我们显示了由大肠杆菌和霍乱弧菌针对霍乱弧菌T6SS效应子TseH的包膜应激反应介导的免疫独立保护。我们证明TseH是PAAR依赖的物种特异性效应子,对气单胞菌物种非常有效,但对它的霍乱弧菌免疫突变体或大肠杆菌却没有。结构分析表明TseH可能是NlpC / P60家族的半胱氨酸内肽酶。我们确定,两个包膜应力响应途径,Rcs和BaeSR,保护E。TseH引起的大肠埃希菌毒性,其机制包括胶囊合成。尽管表达了免疫基因,但两组分系统WigKR(VxrAB)对于保护霍乱弧菌免受自身T6SS侵害至关重要。WigR还调节T6SS的表达,提示其在攻击和防御中起双重作用。这加深了我们对细菌如何在T6SS攻击中生存的理解,并表明针对T6SS的防御代表了主要的选择压力,该压力驱动物种特异性效应子的进化和由包膜应激反应和胶囊合成介导的保护机制。
更新日期:2020-02-24
中文翻译:

包膜应激反应独立于免疫蛋白防御六型分泌系统的攻击。
微生物之间的军备竞赛不仅是武器发展的关键驱动力,也是防御机制发展的关键驱动力。许多革兰氏阴性细菌使用六型分泌系统(T6SS)将毒性效应子直接传递到邻近细胞中。对效应子的防御需要同源的免疫蛋白。但是,这里我们显示了由大肠杆菌和霍乱弧菌针对霍乱弧菌T6SS效应子TseH的包膜应激反应介导的免疫独立保护。我们证明TseH是PAAR依赖的物种特异性效应子,对气单胞菌物种非常有效,但对它的霍乱弧菌免疫突变体或大肠杆菌却没有。结构分析表明TseH可能是NlpC / P60家族的半胱氨酸内肽酶。我们确定,两个包膜应力响应途径,Rcs和BaeSR,保护E。TseH引起的大肠埃希菌毒性,其机制包括胶囊合成。尽管表达了免疫基因,但两组分系统WigKR(VxrAB)对于保护霍乱弧菌免受自身T6SS侵害至关重要。WigR还调节T6SS的表达,提示其在攻击和防御中起双重作用。这加深了我们对细菌如何在T6SS攻击中生存的理解,并表明针对T6SS的防御代表了主要的选择压力,该压力驱动物种特异性效应子的进化和由包膜应激反应和胶囊合成介导的保护机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号