当前位置:
X-MOL 学术
›
JACC Cardiovasc. Inte.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Bicuspid Aortic Valve Stenosis.
JACC: Cardiovascular Interventions ( IF 11.3 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.jcin.2020.02.008 Ron Waksman 1 , Paige E Craig 1 , Rebecca Torguson 1 , Federico M Asch 2 , Gaby Weissman 3 , Daniel Ruiz 1 , Paul Gordon 4 , Afshin Ehsan 5 , Puja Parikh 6 , Thomas Bilfinger 7 , Robert Levitt 8 , Chiwon Hahn 9 , David Roberts 10 , Michael Ingram 10 , Nicholas Hanna 11 , George Comas 12 , Cheng Zhang 1 , Itsik Ben-Dor 1 , Lowell F Satler 1 , Hector M Garcia-Garcia 1 , Christian Shults 13 , Toby Rogers 14
JACC: Cardiovascular Interventions ( IF 11.3 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.jcin.2020.02.008 Ron Waksman 1 , Paige E Craig 1 , Rebecca Torguson 1 , Federico M Asch 2 , Gaby Weissman 3 , Daniel Ruiz 1 , Paul Gordon 4 , Afshin Ehsan 5 , Puja Parikh 6 , Thomas Bilfinger 7 , Robert Levitt 8 , Chiwon Hahn 9 , David Roberts 10 , Michael Ingram 10 , Nicholas Hanna 11 , George Comas 12 , Cheng Zhang 1 , Itsik Ben-Dor 1 , Lowell F Satler 1 , Hector M Garcia-Garcia 1 , Christian Shults 13 , Toby Rogers 14
Affiliation
|
OBJECTIVES
The aim of this study was to evaluate clinical outcomes and transcatheter heart valve hemodynamic parameters after transcatheter aortic valve replacement (TAVR) in low-risk patients with bicuspid aortic stenosis (AS).
BACKGROUND
TAVR is approved for low-risk patients in the United States. However, patients with bicuspid AS were excluded from the randomized cohorts of the pivotal low-risk trials.
METHODS
The LRT (Low Risk TAVR) trial was an investigator-initiated, prospective, multicenter study and was the first and only U.S. Food and Drug Administration-approved investigational device exemption trial to evaluate the feasibility of TAVR with either balloon-expandable or self-expanding valves in low-risk patients with bicuspid AS. The primary endpoint was all-cause mortality at 30 days. Baseline and follow-up echocardiography and computed tomography to detect leaflet thickening were analyzed in an independent core laboratory.
RESULTS
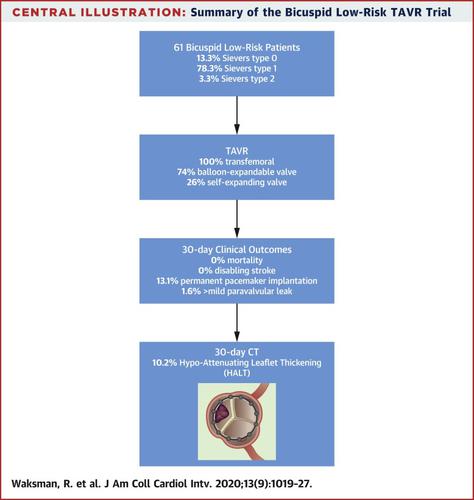
Sixty-one low-risk patients with symptomatic, severe AS and bicuspid aortic valves (78.3% Sievers type 1 morphology) underwent TAVR at 6 centers from 2016 to 2019. The mean age was 68.6 years, and 42.6% were men. At 30 days, there was zero mortality and no disabling strokes. The rate of new permanent pacemaker implantation was 13.1%; just 1 patient had a moderate paravalvular leak at 30 days. Hypoattenuated leaflet thickening was observed in 10% of patients at 30 days.
CONCLUSIONS
TAVR appears to be safe in patients with bicuspid AS, with short length of hospital stay, zero mortality, and no disabling strokes at 30 days. Subclinical leaflet thrombosis was observed in a minority of patients at 30 days but did not appear to be associated with clinical events.
中文翻译:

有症状的严重双尖瓣主动脉瓣狭窄的低风险患者经导管主动脉瓣置换术。
目的本研究旨在评估低风险双尖瓣主动脉瓣狭窄(AS)患者的经导管主动脉瓣置换术(TAVR)后的临床结局和经导管心脏瓣膜血流动力学参数。背景技术TAVR在美国被批准用于低危患者。但是,二尖瓣AS患者被排除在关键性低风险试验的随机队列中。方法LRT(低风险TAVR)试验是一项由研究人员发起的前瞻性多中心研究,并且是美国食品药品监督管理局批准的首个也是唯一的一项通过球囊可扩张或自测技术评估TAVR可行性的试验装置豁免试验。二尖瓣AS低危患者的心脏瓣膜扩张。主要终点是30天时的全因死亡率。在独立的核心实验室中分析了基线和后续超声心动图以及计算机断层扫描以检测小叶增厚。结果2016年至2019年,在6个中心对61例有症状,严重AS和双尖瓣主动脉瓣低危患者(78.3%Sievers 1型形态)进行了TAVR。平均年龄为68.6岁,男性为42.6%。在第30天,死亡率为零,无致残性卒中。新的永久性起搏器植入率为13.1%;仅有1名患者在30天出现中度瓣周漏。30天时,在10%的患者中观察到低衰减的小叶增厚。结论TAVR在二尖瓣AS患者中似乎是安全的,住院时间短,死亡率为零,在30天时无致残性卒中。
更新日期:2020-02-24
中文翻译:

有症状的严重双尖瓣主动脉瓣狭窄的低风险患者经导管主动脉瓣置换术。
目的本研究旨在评估低风险双尖瓣主动脉瓣狭窄(AS)患者的经导管主动脉瓣置换术(TAVR)后的临床结局和经导管心脏瓣膜血流动力学参数。背景技术TAVR在美国被批准用于低危患者。但是,二尖瓣AS患者被排除在关键性低风险试验的随机队列中。方法LRT(低风险TAVR)试验是一项由研究人员发起的前瞻性多中心研究,并且是美国食品药品监督管理局批准的首个也是唯一的一项通过球囊可扩张或自测技术评估TAVR可行性的试验装置豁免试验。二尖瓣AS低危患者的心脏瓣膜扩张。主要终点是30天时的全因死亡率。在独立的核心实验室中分析了基线和后续超声心动图以及计算机断层扫描以检测小叶增厚。结果2016年至2019年,在6个中心对61例有症状,严重AS和双尖瓣主动脉瓣低危患者(78.3%Sievers 1型形态)进行了TAVR。平均年龄为68.6岁,男性为42.6%。在第30天,死亡率为零,无致残性卒中。新的永久性起搏器植入率为13.1%;仅有1名患者在30天出现中度瓣周漏。30天时,在10%的患者中观察到低衰减的小叶增厚。结论TAVR在二尖瓣AS患者中似乎是安全的,住院时间短,死亡率为零,在30天时无致残性卒中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号