Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Entropic effect and residue specific entropic contribution to the cooperativity in streptavidin–biotin binding
Nanoscale ( IF 6.7 ) Pub Date : 2020/02/24 , DOI: 10.1039/c9nr08380d Yalong Cong 1, 2, 3, 4, 5 , Kaifang Huang 1, 2, 3, 4 , Yuchen Li 1, 2, 3, 4 , Susu Zhong 1, 2, 3, 4 , John Z. H. Zhang 4, 5, 6, 7, 8 , Lili Duan 1, 2, 3, 4
Nanoscale ( IF 6.7 ) Pub Date : 2020/02/24 , DOI: 10.1039/c9nr08380d Yalong Cong 1, 2, 3, 4, 5 , Kaifang Huang 1, 2, 3, 4 , Yuchen Li 1, 2, 3, 4 , Susu Zhong 1, 2, 3, 4 , John Z. H. Zhang 4, 5, 6, 7, 8 , Lili Duan 1, 2, 3, 4
Affiliation
|
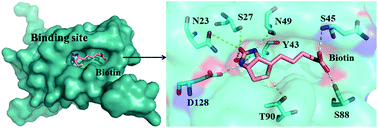
Molecular dynamics (MD) simulations were performed employing the polarized protein-specific charge (PPC) to explore the origin of the cooperativity in streptavidin–biotin systems (wild type, two single mutations and one double-mutation). The results of the experiment found that the existence of cooperativity is mainly the result of the entropic effect. In this study, the entropic contribution to the binding free energy was calculated using the recently developed interaction entropy (IE) method, and computational results are in excellent agreement with the experimental observations and are further verified by the calculation of the thermodynamic integration. Comparison of different force fields in terms of predicted binding strength ordering, cooperativity of energy and the stability of hydrogen bonding suggests that the PPC force field combined IE method is a suitable choice. In addition, the IE method enables us to obtain the residue-specific entropic contributions to the streptavidin–biotin binding affinity and identify ten hot-spot residues providing the dominant contribution to the cooperative binding. Importantly, the overall cooperativity obtained from the ten residues also comes mainly from the entropic effect in our study. The calculation of the potential of mean force shows that the unbinding of streptavidin–biotin is a multi-step process, and each step corresponds to the formation and rupture of the hydrogen bond network. And S45A mutation may increase the rigidity of the linker region, making the flap region relatively difficult to open. The present study provides significant molecular insight into the binding cooperativity of the streptavidin–biotin complex.
中文翻译:

熵效应和残基特异性熵对链霉亲和素-生物素结合的协同作用的贡献
使用极化蛋白特异性电荷(PPC)进行了分子动力学(MD)模拟,以探索链霉亲和素-生物素系统(野生型,两个单突变和一个双突变)中协同作用的起源。实验结果表明,合作性的存在主要是熵效应的结果。在这项研究中,使用最新开发的相互作用熵(IE)方法计算了熵对结合自由能的贡献,计算结果与实验观察值非常吻合,并通过热力学积分的计算得到了进一步验证。根据预测的结合强度顺序比较不同的力场,能量的协同作用和氢键的稳定性表明,PPC力场结合IE方法是合适的选择。另外,IE方法使我们能够获得对链霉亲和素-生物素结合亲和力的残基特异性熵贡献,并鉴定出十个热点残基,它们对协同结合起主要作用。重要的是,从这十个残基获得的整体协同性也主要来自我们研究中的熵效应。平均力势的计算表明,链霉亲和素-生物素的解开是一个多步骤过程,每个步骤都对应于氢键网络的形成和破裂。S45A突变可能会增加接头区域的刚度,从而使折板区域相对难以打开。
更新日期:2020-04-03
中文翻译:

熵效应和残基特异性熵对链霉亲和素-生物素结合的协同作用的贡献
使用极化蛋白特异性电荷(PPC)进行了分子动力学(MD)模拟,以探索链霉亲和素-生物素系统(野生型,两个单突变和一个双突变)中协同作用的起源。实验结果表明,合作性的存在主要是熵效应的结果。在这项研究中,使用最新开发的相互作用熵(IE)方法计算了熵对结合自由能的贡献,计算结果与实验观察值非常吻合,并通过热力学积分的计算得到了进一步验证。根据预测的结合强度顺序比较不同的力场,能量的协同作用和氢键的稳定性表明,PPC力场结合IE方法是合适的选择。另外,IE方法使我们能够获得对链霉亲和素-生物素结合亲和力的残基特异性熵贡献,并鉴定出十个热点残基,它们对协同结合起主要作用。重要的是,从这十个残基获得的整体协同性也主要来自我们研究中的熵效应。平均力势的计算表明,链霉亲和素-生物素的解开是一个多步骤过程,每个步骤都对应于氢键网络的形成和破裂。S45A突变可能会增加接头区域的刚度,从而使折板区域相对难以打开。



























 京公网安备 11010802027423号
京公网安备 11010802027423号