Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.tet.2020.131064 Eiji Tayama , Kazuki Hirano , Souya Baba
|
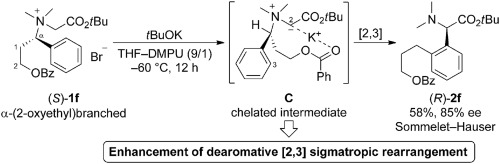
The base-induced Sommelet–Hauser (S–H) rearrangement of N-(α-branched)benzylic glycine ester-derived ammonium salts 1 was investigated. When the α-branched substituent was a simple alkyl, such as a methyl or butyl, desired S–H rearrangement product 2 was obtained in low yield with formation of the [1,2] Stevens rearranged 4 and Hofmann eliminated products 5 and 6. However, when the α-branched substituent had a 2-oxy moiety, such as 2-acetoxyethyl or 2-benzoyloxyethyl, the yields of 2 were improved. These results could be explained by formation of chelated intermediate C that stabilizes the carbanionic ylide, and the subsequent initial dearomative [2,3] sigmatropic rearrangement would be accelerated. The existence of C was supported by mechanistic experiments. This enhancement effect is not very strong or effective; however, it will expand the synthetic usefulness of ammonium ylide rearrangements.
中文翻译:

碱诱导的N-(α-(2-氧乙基)支链)苄基甘氨酸酯衍生的铵盐的Sommelet-Hauser重排通过螯合中间体
研究了碱诱导的N-(α-支链)苄基甘氨酸酯衍生的铵盐1的Sommelet-Hauser重排。当α-支链取代基是一个简单的烷基,例如甲基或丁基时,所需的SH重排产物2低收率地形成了[1,2] Stevens重排4和Hofmann消除的产物5和6。然而,当α-支链取代基有一个-2-氧基部分,例如2-乙酰氧基乙基或2-苯甲酰氧,的产率2得到提高。这些结果可以通过形成螯合的中间体C来解释稳定了碳负离子叶立德,随后的初始脱芳香性[2,3]σ重排将被加速。机械实验证明了C的存在。这种增强效果不是很有效。然而,它将扩大内鎓盐重排的合成用途。


























 京公网安备 11010802027423号
京公网安备 11010802027423号