当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temperature-Dependent Thermodynamic Properties of Amino Acids in Aqueous Imidazolium-Based Ionic Liquid
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.jced.9b00902 Harsh Kumar 1 , Arjuna Katal 1 , Priyank Kumar Sharma 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.jced.9b00902 Harsh Kumar 1 , Arjuna Katal 1 , Priyank Kumar Sharma 1
Affiliation
|
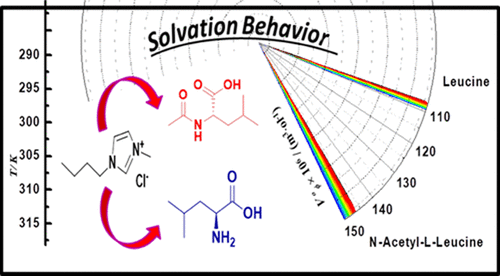
Molecular interactions of amino acids with ionic liquids are significant to study the molecular stability and physicochemical properties of amino acids. Mixtures of amino acids l-leucine and N-acetyl-l-leucine + (0.005, 0.010, 0.030, and 0.050 mol·kg–1) aqueous solution of ionic liquid (1-butyl-3-methylimidazolium chloride) at temperature T = (288.15, 298.15, 308.15, and 318.15 K) have been studied by density and speeds of sound measurements. Volumetric properties have been obtained by utilizing data of density. Volumetric properties include apparent molar volumes, partial molar volumes at infinite dilution, limiting partial molar volumes of transfer at infinite dilution, apparent molar expansibility, etc. Similarly, speeds of sound measurements are utilized to calculate corresponding parameters like apparent molar isentropic compression, Kϕ,s, apparent molar isentropic compression at infinite dilution, Kϕ,s0, and transfer compressibility at infinite dilution ΔKϕ,s0. Results confirmed the predominance of the hydrophilic–hydrophilic interaction over the hydrophobic–hydrophobic interaction in ternary solutions.
中文翻译:

咪唑鎓离子液体中氨基酸的温度依赖性热力学性质
氨基酸与离子液体的分子相互作用对于研究氨基酸的分子稳定性和理化性质具有重要意义。氨基酸的混合物升-亮氨酸和Ñ乙酰基升-亮氨酸+(0.005,0.010,0.030,和0.050摩尔公斤· -1)水性离子液体(1-丁基-3-甲基咪唑鎓氯化物)中的温度溶液Ť=(288.15、298.15、308.15和318.15 K)已通过声音测量的密度和速度进行了研究。通过利用密度数据获得了体积特性。体积特性包括表观摩尔体积,无限稀释时的部分摩尔体积,无限稀释时转移的有限部分摩尔体积,表观摩尔可膨胀性等。类似地,声音测量的速度用于计算相应的参数,例如表观摩尔等熵压缩,K ϕ ,s,无限稀释时的表观摩尔等熵压缩K ϕ,s 0,无限稀释时的传递压缩率ΔK ϕ,s 0。结果证实了三元溶液中亲水-亲水相互作用优于疏水-疏水相互作用。
更新日期:2020-02-21
中文翻译:

咪唑鎓离子液体中氨基酸的温度依赖性热力学性质
氨基酸与离子液体的分子相互作用对于研究氨基酸的分子稳定性和理化性质具有重要意义。氨基酸的混合物升-亮氨酸和Ñ乙酰基升-亮氨酸+(0.005,0.010,0.030,和0.050摩尔公斤· -1)水性离子液体(1-丁基-3-甲基咪唑鎓氯化物)中的温度溶液Ť=(288.15、298.15、308.15和318.15 K)已通过声音测量的密度和速度进行了研究。通过利用密度数据获得了体积特性。体积特性包括表观摩尔体积,无限稀释时的部分摩尔体积,无限稀释时转移的有限部分摩尔体积,表观摩尔可膨胀性等。类似地,声音测量的速度用于计算相应的参数,例如表观摩尔等熵压缩,K ϕ ,s,无限稀释时的表观摩尔等熵压缩K ϕ,s 0,无限稀释时的传递压缩率ΔK ϕ,s 0。结果证实了三元溶液中亲水-亲水相互作用优于疏水-疏水相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号