Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2020-02-22 , DOI: 10.1016/j.apcata.2020.117474 Hongjie Wu , Mingwei Yuan , Jia Huang , Xiaozhong Li , Yan Wang , Jinjun Li , Zhixiong You
|
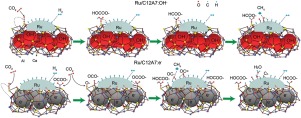
Mayenites (C12A7) encaged with OH- or e- have been synthesized and applied as supports for ruthenium catalyst in CO2 methanation. The catalytic activity of Ru/C12A7:OH- (TOF at 225 °C = 6.04 × 10-2 s-1, Ea =70.9 kJ mol-1) is drastically higher than that of Ru/C12A7:e- (TOF at 225 °C = 2.41 × 10-2 s-1, Ea =94.4 kJ mol-1) and Ru/γ-Al2O3 (TOF at 225 °C = 0.23 × 10-2 s-1, Ea =105.8 kJ mol-1). It has been proposed that the OH- or e- encaged in mayenite promotes the formation of carbonates and bicarbonates from CO2 on the supports. It is supposed that at the interface between Ru and mayenite support the surface carbonates and bicarbonates are hydrogenated into formates, which are further dissociated into *CO adsorbed on Ru. The formed *CO is the intermediate for methane formation and carbon deposition. The different anions (OH- or e-) encaged in the framework of mayenites have been found to influence drastically the ionization potential of Ru. Electrons in mayenite electrides are transferred to Ru metal, thereby enhancing the back donation from Ru to the antibonding orbitals of *CO intermediate. As a consequence, the dissociation of *CO into *C and subsequent into deposition of carbons are accelerated, which is responsible for the inferior catalytic performance of Ru/C12A7:e-. Based on the experimental results, we propose an effective technique for tailoring the catalytic performance of metallic sites supported on mayenites via changing encaged anions.
中文翻译:

Ru / 12CaO∙7Al 2 O 3催化剂上CO 2甲烷化反应:封端阴离子对催化机理的影响
已合成了被OH-或e-包裹的玛雅石(C12A7),并将其用作钌催化剂在CO 2甲烷化中的载体。Ru / C12A7:OH-(225°C下的TOF = 6.04×10 -2 s -1,E a = 70.9 kJ mol -1)的催化活性大大高于Ru / C12A7:e-(TOF在225°C时) 225°C = 2.41×10 -2 s -1,E a = 94.4 kJ mol -1)和Ru / γ- Al 2 O 3(225°C时的TOF = 0.23×10 -2 s -1,E a = 105.8 kJ摩尔-1)。有人提出,钙铝石中的OH-或e-会促进由CO 2形成碳酸盐和碳酸氢盐在支撑上。据推测,在Ru和钙铝石载体之间的界面处,碳酸盐和碳酸氢盐的表面被氢化成甲酸盐,然后进一步分解成吸附在Ru上的* CO。形成的* CO是甲烷形成和碳沉积的中间体。发现在钙铝石框架内的不同阴离子(OH-或e-)会极大地影响Ru的电离势。钙铝石驻极体中的电子被转移到Ru金属上,从而增强了Ru向* CO中间物的反键轨道的背电荷。结果,加速了* CO分解成* C以及随后沉积成碳,这是Ru / C12A7:e-催化性能较差的原因。根据实验结果,


























 京公网安备 11010802027423号
京公网安备 11010802027423号