当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer's disease.
Aging Cell ( IF 7.8 ) Pub Date : 2020-02-22 , DOI: 10.1111/acel.13118 Carlos J Rodriguez-Ortiz 1 , Gilberto Aleph Prieto 2, 3 , Alessandra C Martini 2 , Stefania Forner 2 , Laura Trujillo-Estrada 2 , Frank M LaFerla 2 , David Baglietto-Vargas 2 , Carl W Cotman 2, 4 , Masashi Kitazawa 1
Aging Cell ( IF 7.8 ) Pub Date : 2020-02-22 , DOI: 10.1111/acel.13118 Carlos J Rodriguez-Ortiz 1 , Gilberto Aleph Prieto 2, 3 , Alessandra C Martini 2 , Stefania Forner 2 , Laura Trujillo-Estrada 2 , Frank M LaFerla 2 , David Baglietto-Vargas 2 , Carl W Cotman 2, 4 , Masashi Kitazawa 1
Affiliation
|
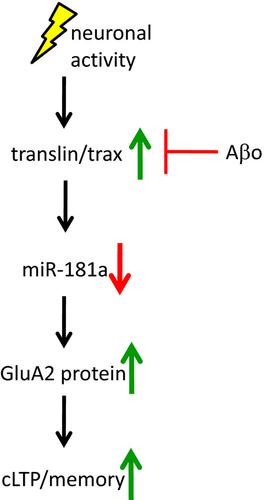
MicroRNAs play a pivotal role in rapid, dynamic, and spatiotemporal modulation of synaptic functions. Among them, recent emerging evidence highlights that microRNA‐181a (miR‐181a) is particularly abundant in hippocampal neurons and controls the expression of key plasticity‐related proteins at synapses. We have previously demonstrated that miR‐181a was upregulated in the hippocampus of a mouse model of Alzheimer's disease (AD) and correlated with reduced levels of plasticity‐related proteins. Here, we further investigated the underlying mechanisms by which miR‐181a negatively modulated synaptic plasticity and memory. In primary hippocampal cultures, we found that an activity‐dependent upregulation of the microRNA‐regulating protein, translin, correlated with reduction of miR‐181a upon chemical long‐term potentiation (cLTP), which induced upregulation of GluA2, a predicted target for miR‐181a, and other plasticity‐related proteins. Additionally, Aβ treatment inhibited cLTP‐dependent induction of translin and subsequent reduction of miR‐181a, and cotreatment with miR‐181a antagomir effectively reversed the effects elicited by Aβ but did not rescue translin levels, suggesting that the activity‐dependent upregulation of translin was upstream of miR‐181a. In mice, a learning episode markedly decreased miR‐181a in the hippocampus and raised the protein levels of GluA2. Lastly, we observed that inhibition of miR‐181a alleviated memory deficits and increased GluA2 and GluA1 levels, without restoring translin, in the 3xTg‐AD model. Taken together, our results indicate that miR‐181a is a major negative regulator of the cellular events that underlie synaptic plasticity and memory through AMPA receptors, and importantly, Aβ disrupts this process by suppressing translin and leads to synaptic dysfunction and memory impairments in AD.
中文翻译:

miR-181a 负向调节海马培养物中的突触可塑性,其抑制作用可挽救阿尔茨海默病小鼠模型中的记忆缺陷。
MicroRNA 在突触功能的快速、动态和时空调节中发挥着关键作用。其中,最近新出现的证据强调,microRNA-181a (miR-181a) 在海马神经元中特别丰富,并控制突触中关键可塑性相关蛋白的表达。我们之前已经证明 miR-181a 在阿尔茨海默病 (AD) 小鼠模型的海马中上调,并与可塑性相关蛋白水平降低相关。在这里,我们进一步研究了 miR-181a 负调节突触可塑性和记忆的潜在机制。在原代海马培养物中,我们发现 microRNA 调节蛋白 translin 的活性依赖性上调与化学长时程增强 (cLTP) 后 miR-181a 的减少相关,它诱导了 GluA2(miR-181a 的预测靶标)和其他可塑性相关蛋白的上调。此外,Aβ 处理抑制了 cLTP 依赖的 translin 诱导和随后 miR-181a 的减少,并且与 miR-181a antagomir 共同处理有效地逆转了 Aβ 引起的作用,但没有挽救 translin 水平,表明 translin 的活性依赖性上调是miR-181a 的上游。在小鼠中,学习阶段显着降低了海马体中的 miR-181a,并提高了 GluA2 的蛋白质水平。最后,我们观察到在 3xTg-AD 模型中,抑制 miR-181a 减轻了记忆缺陷并增加了 GluA2 和 GluA1 水平,而没有恢复 translin。综合起来,
更新日期:2020-02-22
中文翻译:

miR-181a 负向调节海马培养物中的突触可塑性,其抑制作用可挽救阿尔茨海默病小鼠模型中的记忆缺陷。
MicroRNA 在突触功能的快速、动态和时空调节中发挥着关键作用。其中,最近新出现的证据强调,microRNA-181a (miR-181a) 在海马神经元中特别丰富,并控制突触中关键可塑性相关蛋白的表达。我们之前已经证明 miR-181a 在阿尔茨海默病 (AD) 小鼠模型的海马中上调,并与可塑性相关蛋白水平降低相关。在这里,我们进一步研究了 miR-181a 负调节突触可塑性和记忆的潜在机制。在原代海马培养物中,我们发现 microRNA 调节蛋白 translin 的活性依赖性上调与化学长时程增强 (cLTP) 后 miR-181a 的减少相关,它诱导了 GluA2(miR-181a 的预测靶标)和其他可塑性相关蛋白的上调。此外,Aβ 处理抑制了 cLTP 依赖的 translin 诱导和随后 miR-181a 的减少,并且与 miR-181a antagomir 共同处理有效地逆转了 Aβ 引起的作用,但没有挽救 translin 水平,表明 translin 的活性依赖性上调是miR-181a 的上游。在小鼠中,学习阶段显着降低了海马体中的 miR-181a,并提高了 GluA2 的蛋白质水平。最后,我们观察到在 3xTg-AD 模型中,抑制 miR-181a 减轻了记忆缺陷并增加了 GluA2 和 GluA1 水平,而没有恢复 translin。综合起来,

























 京公网安备 11010802027423号
京公网安备 11010802027423号