当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
D–π–A-type triphenylamine dye covalent-functionalized g-C3N4 for highly efficient photocatalytic hydrogen evolution
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020/02/21 , DOI: 10.1039/d0cy00013b Chao Zhang 1, 2, 3, 4 , Jiandong Liu 1, 2, 3, 4 , Xingliang Liu 1, 2, 3, 4 , Shiai Xu 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020/02/21 , DOI: 10.1039/d0cy00013b Chao Zhang 1, 2, 3, 4 , Jiandong Liu 1, 2, 3, 4 , Xingliang Liu 1, 2, 3, 4 , Shiai Xu 1, 2, 3, 4, 5
Affiliation
|
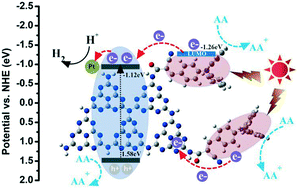
Two novel covalent bond photocatalysts, namely, g-C3N4NSs/TC1 and g-C3N4NSs/TC2 were prepared for the first time via the generation of an amide covalent bond between g-C3N4 nanosheets (g-C3N4NSs) and acyl chloride synthesized from triphenylamine-type dyes, 2-cyano-3-(4-(diphenylamino)phenyl)acrylic acid (TC1) or 3,3′-(4,4′-(phenylazanediyl)bis(4,1-phenylene))bis(2-cy-anoacrylic acid) (TC2). The experimental results indicated that triphenylamine D–π–A-type organic dye moieties covalently bonded to g-C3N4 successfully and the spectral response regions of both g-C3N4NSs/TC1 and g-C3N4NSs/TC2 could be extended from ∼460 nm to more than 600 nm. Due to the introduced amide covalent bond, the constructed g-C3N4NSs/TC1 photocatalyst showed remarkably enhanced H2 evolution activity with about 73 555.8 μmol h−1 g−1 and exhibited high apparent quantum yields (AQY) of 35.2%, 27.1%, 16.9% and 5.4% at 420 nm, 500 nm, 520 nm and 600 nm monochromatic light, respectively. Compared with the results for pure g-C3N4 NSs, about 100 times higher H2 production rate and 978 times higher AQY value were obtained for g-C3N4NSs/TC1 with the amide covalent bond under visible light irradiation. Compared to g-C3N4NSs/TC1, g-C3N4NSs/TC2 exhibited slightly lower H2 evolution activity with about 70 986.8 μmol h−1 g−1. Combining the experimental and density functional theory (DFT) calculation results, a possible mechanism can be conjectured that the electronic and geometric structures of g-C3N4NSs/TC1 are more conducive to the charge separation and the unidirectionality of electron transport in the excited state than those of g-C3N4NSs/TC2. The research reveals that this introduction of simple triphenylamine-type dyes into the surface of g-C3N4via covalent links results in a strong covalent interaction between functionalized moieties and g-C3N4 to synergistically improve the electronic and optical properties and photocatalytic performance. The present work highlights the potential use of organic dye covalent-functionalized g-C3N4via post modification as a novel photocatalyst in applications such as H2 production.
中文翻译:

D–π–A型三苯胺染料共价官能化的g-C3N4用于高效光催化氢的释放
通过在gC 3 N 4纳米片(gC 3 N 4 NSs)之间生成酰胺共价键,首次制备了两种新型的共价键光催化剂gC 3 N 4 NSs / TC1和gC 3 N 4 NSs / TC2。和由三苯胺型染料,2-氰基-3-(4-(二苯氨基)苯基)丙烯酸(TC1)或3,3'-(4,4'-(苯基氮杂二基)双(4,1-)合成的酰氯亚苯基))双(2-氰基丙烯酸)(TC2)。实验结果表明,三苯胺D–π–A型有机染料部分成功地与gC 3 N 4共价键合,并且可以扩展gC 3 N 4 NSs / TC1和gC 3 N 4 NSs / TC2的光谱响应区域。约460 nm至600 nm以上 由于引入了酰胺共价键,所构建的gC 3 N 4 NSs / TC1光催化剂显示出显着增强的H 2析出活性,约为73 555.8μmolh -1 g -1并在420 nm,500 nm,520 nm和600 nm单色光下分别表现出35.2%,27.1%,16.9%和5.4%的高表观量子产率。与纯gC 3 N 4 NSs的结果相比,在可见光照射下,具有酰胺共价键的gC 3 N 4 NSs / TC1的H 2生产率提高了约100倍,AQY值提高了978倍。与gC 3 N 4 NSs / TC1相比,gC 3 N 4 NSs / TC2的H 2释放活性略低,约为70 986.8μmolh -1g -1。结合实验和密度泛函理论(DFT)的计算结果,可以推测出一种可能的机制,即gC 3 N 4 NSs / TC1的电子和几何结构更有利于激发状态下的电荷分离和电子传输的单向性比gC 3 N 4 NSs / TC2高。研究表明,通过共价键将简单的三苯胺型染料引入gC 3 N 4的表面导致官能化部分与gC 3 N 4之间的强共价相互作用。协同改善电子和光学性能以及光催化性能。本工作强调了通过后修饰将有机染料共价官能化的gC 3 N 4用作新型光催化剂在H 2生产中的潜在用途。
更新日期:2020-03-26
中文翻译:

D–π–A型三苯胺染料共价官能化的g-C3N4用于高效光催化氢的释放
通过在gC 3 N 4纳米片(gC 3 N 4 NSs)之间生成酰胺共价键,首次制备了两种新型的共价键光催化剂gC 3 N 4 NSs / TC1和gC 3 N 4 NSs / TC2。和由三苯胺型染料,2-氰基-3-(4-(二苯氨基)苯基)丙烯酸(TC1)或3,3'-(4,4'-(苯基氮杂二基)双(4,1-)合成的酰氯亚苯基))双(2-氰基丙烯酸)(TC2)。实验结果表明,三苯胺D–π–A型有机染料部分成功地与gC 3 N 4共价键合,并且可以扩展gC 3 N 4 NSs / TC1和gC 3 N 4 NSs / TC2的光谱响应区域。约460 nm至600 nm以上 由于引入了酰胺共价键,所构建的gC 3 N 4 NSs / TC1光催化剂显示出显着增强的H 2析出活性,约为73 555.8μmolh -1 g -1并在420 nm,500 nm,520 nm和600 nm单色光下分别表现出35.2%,27.1%,16.9%和5.4%的高表观量子产率。与纯gC 3 N 4 NSs的结果相比,在可见光照射下,具有酰胺共价键的gC 3 N 4 NSs / TC1的H 2生产率提高了约100倍,AQY值提高了978倍。与gC 3 N 4 NSs / TC1相比,gC 3 N 4 NSs / TC2的H 2释放活性略低,约为70 986.8μmolh -1g -1。结合实验和密度泛函理论(DFT)的计算结果,可以推测出一种可能的机制,即gC 3 N 4 NSs / TC1的电子和几何结构更有利于激发状态下的电荷分离和电子传输的单向性比gC 3 N 4 NSs / TC2高。研究表明,通过共价键将简单的三苯胺型染料引入gC 3 N 4的表面导致官能化部分与gC 3 N 4之间的强共价相互作用。协同改善电子和光学性能以及光催化性能。本工作强调了通过后修饰将有机染料共价官能化的gC 3 N 4用作新型光催化剂在H 2生产中的潜在用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号