当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A mechanism of metastable sulfur speciation and the adsorption on a gold surface in the presence of sulfidic ore and lead in cyanide medium
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.hydromet.2020.105294 Rina Kim , Ahmad Ghahreman
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.hydromet.2020.105294 Rina Kim , Ahmad Ghahreman
|
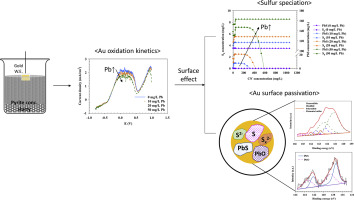
Abstract Metallic gold or gold minerals have high affinity towards aqueous sulfur species during gold leaching process in cyanide solutions, thus the gold surface can often become passivated by the reaction between the different sulfur species and gold. During the cyanide leaching of sulfidic gold ores, the sulfide minerals dissolve into the leach solution and produce metastable sulfur species, such as sulfide, bisulfide, polysulfide, elemental sulfur, and thiocyanate. Most of the above sulfur species can directly passivate the gold surface, and significantly reduce the gold leaching efficiency. This paper pertains to studying the sulfur species formation in cyanide solutions and their adsorption chemistry and mechanism on gold surface. It was found that the lead salts, often added to the cyanidation process to improve the gold leaching efficiency, act as a sulfide oxidant and accelerates its oxidation to different metastable sulfur species. It was shown by electrochemical linear sweep voltammetry (LSV) tests that the gold oxidation current density, i.e. oxidation kinetics, was decreased 32%, from 2.20 to 1.50 mA/cm2, in the presence of a high sulfide ore by increasing the amount of lead salt from 0 to 50 mg/L at 300 mg/L free cyanide (300 mg/L CN). Monosulfide, disulfide and polysulfide sulfur species were detected on the gold surface, by X-ray photoelectron spectroscopy (XPS) method, confirming the adsorption of different sulfur species on the gold surface. At high concentration of added lead, 50 mg/L Pb2+, even elemental sulfur was shown to adsorb on the gold surface. The formation of different sulfur species was explored by thermodynamic modeling, which showed very good agreement with the results of the XPS study. This study helps to develop a better insight on the mechanism of gold surface passivation caused by sulfur species and the findings help to reduce gold passivation by better control of the process condition.
中文翻译:

氰化物介质中硫化矿和铅存在下亚稳态硫形态形成和金表面吸附的机理
摘要 金属金或金矿物在氰化物溶液中浸金过程中对含水硫物种具有高亲和力,因此不同硫物种与金之间的反应往往会使金表面钝化。在硫化金矿石氰化浸出过程中,硫化物矿物溶解在浸出液中并产生亚稳硫物种,如硫化物、二硫化物、多硫化物、元素硫和硫氰酸盐。上述硫磺种类大多能直接钝化金表面,显着降低浸金效率。本文主要研究氰化物溶液中硫的形成及其在金表面的吸附化学和机理。研究发现,通常在氰化过程中加入铅盐以提高浸金效率,充当硫化物氧化剂并加速其氧化成不同的亚稳态硫物种。电化学线性扫描伏安法 (LSV) 测试表明,在高硫化矿存在下,通过增加铅含量,金氧化电流密度,即氧化动力学,从 2.20 到 1.50 mA/cm2 降低了 32% 0 至 50 mg/L 的盐,300 mg/L 游离氰化物 (300 mg/L CN)。通过 X 射线光电子能谱 (XPS) 方法在金表面检测到单硫化物、二硫化物和多硫化物硫物种,证实了不同硫物种在金表面的吸附。在高浓度添加的铅(50 mg/L Pb2+)下,甚至元素硫也显示出吸附在金表面上。通过热力学模型探索了不同硫物种的形成,这与 XPS 研究的结果非常吻合。这项研究有助于更好地了解由硫物质引起的金表面钝化的机制,这些发现有助于通过更好地控制工艺条件来减少金钝化。
更新日期:2020-05-01
中文翻译:

氰化物介质中硫化矿和铅存在下亚稳态硫形态形成和金表面吸附的机理
摘要 金属金或金矿物在氰化物溶液中浸金过程中对含水硫物种具有高亲和力,因此不同硫物种与金之间的反应往往会使金表面钝化。在硫化金矿石氰化浸出过程中,硫化物矿物溶解在浸出液中并产生亚稳硫物种,如硫化物、二硫化物、多硫化物、元素硫和硫氰酸盐。上述硫磺种类大多能直接钝化金表面,显着降低浸金效率。本文主要研究氰化物溶液中硫的形成及其在金表面的吸附化学和机理。研究发现,通常在氰化过程中加入铅盐以提高浸金效率,充当硫化物氧化剂并加速其氧化成不同的亚稳态硫物种。电化学线性扫描伏安法 (LSV) 测试表明,在高硫化矿存在下,通过增加铅含量,金氧化电流密度,即氧化动力学,从 2.20 到 1.50 mA/cm2 降低了 32% 0 至 50 mg/L 的盐,300 mg/L 游离氰化物 (300 mg/L CN)。通过 X 射线光电子能谱 (XPS) 方法在金表面检测到单硫化物、二硫化物和多硫化物硫物种,证实了不同硫物种在金表面的吸附。在高浓度添加的铅(50 mg/L Pb2+)下,甚至元素硫也显示出吸附在金表面上。通过热力学模型探索了不同硫物种的形成,这与 XPS 研究的结果非常吻合。这项研究有助于更好地了解由硫物质引起的金表面钝化的机制,这些发现有助于通过更好地控制工艺条件来减少金钝化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号