当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
SPLIT: Stable Protein Coacervation Using a Light Induced Transition.
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-02-28 , DOI: 10.1021/acssynbio.9b00503 Ellen H Reed 1 , Benjamin S Schuster 2 , Matthew C Good 3 , Daniel A Hammer 1, 4
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-02-28 , DOI: 10.1021/acssynbio.9b00503 Ellen H Reed 1 , Benjamin S Schuster 2 , Matthew C Good 3 , Daniel A Hammer 1, 4
Affiliation
|
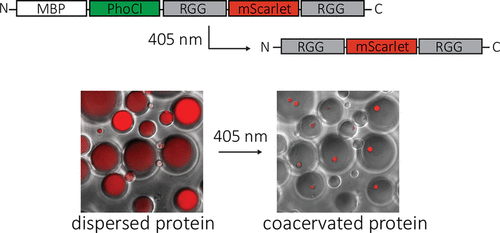
Protein coacervates serve as hubs to concentrate and sequester proteins and nucleotides and thus function as membraneless organelles to manipulate cell physiology. We have engineered a coacervating protein to create tunable, synthetic membraneless organelles that assemble in response to a single pulse of light. Coacervation is driven by the intrinsically disordered RGG domain from the protein LAF-1, and opto-responsiveness is coded by the protein PhoCl, which cleaves in response to 405 nm light. We developed a fusion protein containing a solubilizing maltose-binding protein domain, PhoCl, and two copies of the RGG domain. Several seconds of illumination at 405 nm is sufficient to cleave PhoCl, removing the solubilization domain and enabling RGG-driven coacervation within minutes in cellular-sized water-in-oil emulsions. An optimized version of this system displayed light-induced coacervation in Saccharomyces cerevisiae. The methods described here provide novel strategies for inducing protein phase separation using light.
中文翻译:

分裂:使用光诱导的跃迁进行稳定的蛋白质凝聚。
蛋白质凝聚层充当集中和隔离蛋白质和核苷酸的枢纽,因此起无膜细胞器的作用,以操纵细胞生理。我们已经设计出一种凝聚蛋白来创建可调谐的合成无膜细胞器,这些细胞器会响应单个光脉冲而组装。凝聚由来自蛋白LAF-1的内在无序的RGG域驱动,而光响应性则由蛋白PhoCl编码,该蛋白响应405 nm的光而裂解。我们开发了一种融合蛋白,其中包含一个可溶性麦芽糖结合蛋白结构域,PhoCl和两个RGG结构域副本。在405 nm处照射几秒钟足以裂解PhoCl,除去增溶结构域,并在数分钟内在细胞大小的油包水乳液中实现RGG驱动的凝聚。该系统的优化版本在酿酒酵母中显示出光诱导的凝聚。这里描述的方法提供了使用光诱导蛋白质相分离的新策略。
更新日期:2020-02-28
中文翻译:

分裂:使用光诱导的跃迁进行稳定的蛋白质凝聚。
蛋白质凝聚层充当集中和隔离蛋白质和核苷酸的枢纽,因此起无膜细胞器的作用,以操纵细胞生理。我们已经设计出一种凝聚蛋白来创建可调谐的合成无膜细胞器,这些细胞器会响应单个光脉冲而组装。凝聚由来自蛋白LAF-1的内在无序的RGG域驱动,而光响应性则由蛋白PhoCl编码,该蛋白响应405 nm的光而裂解。我们开发了一种融合蛋白,其中包含一个可溶性麦芽糖结合蛋白结构域,PhoCl和两个RGG结构域副本。在405 nm处照射几秒钟足以裂解PhoCl,除去增溶结构域,并在数分钟内在细胞大小的油包水乳液中实现RGG驱动的凝聚。该系统的优化版本在酿酒酵母中显示出光诱导的凝聚。这里描述的方法提供了使用光诱导蛋白质相分离的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号