Nature Metabolism ( IF 20.8 ) Pub Date : 2020-02-20 , DOI: 10.1038/s42255-020-0169-x Alisson C. Cardoso , Nicholas T. Lam , Jainy J. Savla , Yuji Nakada , Ana Helena M. Pereira , Abdallah Elnwasany , Ivan Menendez-Montes , Emily L. Ensley , Ursa Bezan Petric , Gaurav Sharma , A. Dean Sherry , Craig R. Malloy , Chalermchai Khemtong , Michael T. Kinter , Wilson Lek Wen Tan , Chukwuemeka G. Anene-Nzelu , Roger Sik-Yin Foo , Ngoc Uyen Nhi Nguyen , Shujuan Li , Mahmoud Salama Ahmed , Waleed M. Elhelaly , Salim Abdisalaam , Aroumougame Asaithamby , Chao Xing , Mohammed Kanchwala , Gonçalo Vale , Kaitlyn M. Eckert , Matthew A. Mitsche , Jeffrey G. McDonald , Joseph A. Hill , Linzhang Huang , Philip W. Shaul , Luke I. Szweda , Hesham A. Sadek
|
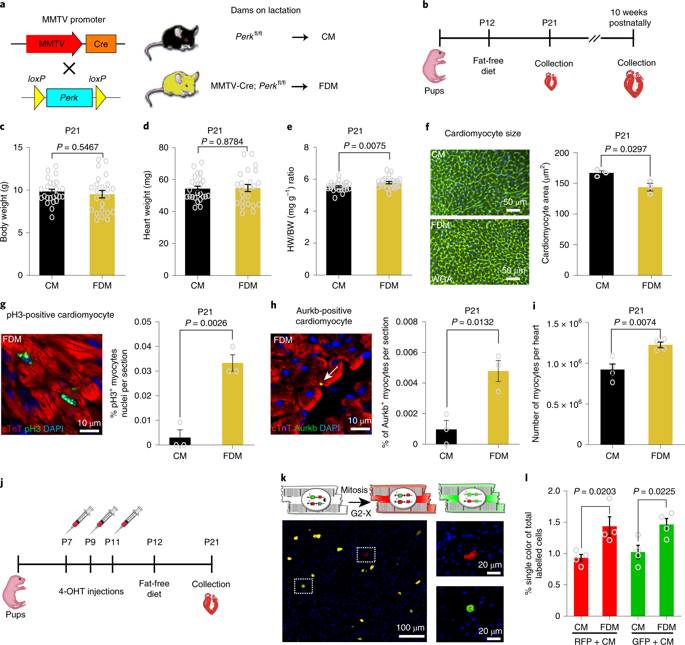
The neonatal mammalian heart is capable of regeneration for a brief window of time after birth. However, this regenerative capacity is lost within the first week of life, which coincides with a postnatal shift from anaerobic glycolysis to mitochondrial oxidative phosphorylation, particularly towards fatty-acid utilization. Despite the energy advantage of fatty-acid beta-oxidation, cardiac mitochondria produce elevated rates of reactive oxygen species when utilizing fatty acids, which is thought to play a role in cardiomyocyte cell-cycle arrest through induction of DNA damage and activation of DNA-damage response (DDR) pathway. Here we show that inhibiting fatty-acid utilization promotes cardiomyocyte proliferation in the postnatal heart. First, neonatal mice fed fatty-acid-deficient milk showed prolongation of the postnatal cardiomyocyte proliferative window; however, cell-cycle arrest eventually ensued. Next, we generated a tamoxifen-inducible cardiomyocyte-specific pyruvate dehydrogenase kinase 4 (PDK4) knockout mouse model to selectively enhance oxidation of glycolytically derived pyruvate in cardiomyocytes. Conditional PDK4 deletion resulted in an increase in pyruvate dehydrogenase activity and consequently an increase in glucose relative to fatty-acid oxidation. Loss of PDK4 also resulted in decreased cardiomyocyte size, decreased DNA damage and expression of DDR markers and an increase in cardiomyocyte proliferation. Following myocardial infarction, inducible deletion of PDK4 improved left ventricular function and decreased remodelling. Collectively, inhibition of fatty-acid utilization in cardiomyocytes promotes proliferation, and may be a viable target for cardiac regenerative therapies.
中文翻译:

线粒体底物的利用调节心肌细胞周期的进程
新生的哺乳动物心脏能够在出生后短暂的时间内再生。然而,这种再生能力在生命的第一周内丧失了,这与出生后从无氧糖酵解转变为线粒体氧化磷酸化,尤其是向脂肪酸利用的转变相吻合。尽管脂肪酸β-氧化具有能量优势,但利用脂肪酸时,心脏线粒体会产生较高的活性氧比率,这被认为通过诱导DNA损伤和激活DNA损伤在心肌细胞周期停滞中发挥作用响应(DDR)途径。在这里,我们表明抑制脂肪酸的利用会促进出生后心脏中的心肌细胞增殖。第一,喂养缺乏脂肪酸的牛奶的新生小鼠表现出出生后心肌细胞增殖窗口的延长;然而,细胞周期的逮捕最终发生了。接下来,我们生成了他莫昔芬诱导型心肌细胞特异性丙酮酸脱氢酶激酶4(PDK4)敲除小鼠模型,以选择性增强心肌细胞中糖酵解性丙酮酸的氧化。有条件的PDK4缺失导致丙酮酸脱氢酶活性增加,因此相对于脂肪酸氧化,葡萄糖增加。PDK4的丢失还导致心肌细胞大小减少,DNA损伤和DDR标记物表达减少以及心肌细胞增殖增加。心肌梗塞后,PDK4的诱导型缺失可改善左心室功能并减少重塑。总的来说,



























 京公网安备 11010802027423号
京公网安备 11010802027423号