当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Molecular Mechanism of Polymer Formation of Farnesylated Human Guanylate-binding Protein 1.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jmb.2020.02.009 Linda Sistemich 1 , Miriam Kutsch 2 , Benjamin Hämisch 3 , Ping Zhang 1 , Sergii Shydlovskyi 1 , Nathalie Britzen-Laurent 4 , Michael Stürzl 4 , Klaus Huber 3 , Christian Herrmann 1
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jmb.2020.02.009 Linda Sistemich 1 , Miriam Kutsch 2 , Benjamin Hämisch 3 , Ping Zhang 1 , Sergii Shydlovskyi 1 , Nathalie Britzen-Laurent 4 , Michael Stürzl 4 , Klaus Huber 3 , Christian Herrmann 1
Affiliation
|
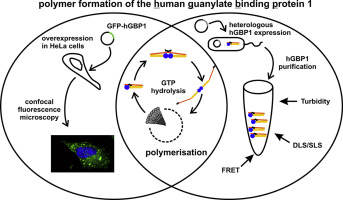
The human guanylate-binding protein 1 (hGBP1) belongs to the dynamin superfamily proteins and represents a key player in the innate immune response. Farnesylation at the C-terminus is required for hGBP1's activity against microbial pathogens, as well as for its antiproliferative and antitumor activity. The farnesylated hGBP1 (hGBP1fn) retains many characteristics of the extensively studied nonfarnesylated protein and gains additional abilities like binding to lipid membranes and formation of hGBP1fn polymers. These polymers are believed to serve as a protein depot, making the enzyme immediately available to fight the invasion of intracellular pathogens. Here we study the molecular mechanism of hGBP1 polymer formation as it is a crucial state of this enzyme, allowing for a rapid response demanded by the biological function. We employ Förster resonance energy transfer in order to trace intra and intermolecular distance changes of protein domains. Light scattering techniques yield deep insights into the changes in size and shape. The GTP hydrolysis driven cycling between a closed, farnesyl moiety hidden state and an opened, farnesyl moiety exposed state represents the first phase, preparing the molecule for polymerization. Within the second phase of polymer growth, opened hGBP1 molecules can be incorporated in the growing polymer where the opened structure is stabilized, similar to a surfactant molecule in a micelle, pointing the farnesyl moieties into the hydrophobic center and positioning the head groups at the periphery of the polymer. We contribute the molecular mechanism of polymer formation, paving the ground for a detailed understanding of hGBP1 function.
中文翻译:

法呢基化人鸟苷酸结合蛋白1形成聚合物的分子机理。
人鸟苷酸结合蛋白1(hGBP1)属于dynamin超家族蛋白,代表先天免疫应答中的关键角色。hGBP1抗微生物病原体的活性及其抗增殖和抗肿瘤活性需要C端的法呢基化。法呢基化的hGBP1(hGBP1fn)保留了广泛研究的非法呢基化蛋白的许多特征,并获得了额外的功能,例如与脂质膜结合和hGBP1fn聚合物的形成。据信这些聚合物可作为蛋白质贮库,使该酶可立即用于抵抗细胞内病原体的入侵。在这里,我们研究hGBP1聚合物形成的分子机制,因为它是该酶的关键状态,可以实现生物学功能所需的快速响应。我们使用福斯特共振能量转移来追踪蛋白质结构域内和分子间的距离变化。光散射技术可深入了解尺寸和形状的变化。在封闭的法呢基部分隐藏状态和开放的法呢基部分暴露状态之间,由GTP水解驱动的循环代表第一相,为聚合准备分子。在聚合物生长的第二阶段中,可以将打开的hGBP1分子掺入到增长的聚合物中,在该聚合物中,打开的结构得以稳定,类似于胶束中的表面活性剂分子,将法呢基部分指向疏水中心并将头基定位在外围聚合物。我们贡献了聚合物形成的分子机制,为对hGBP1功能的详细了解奠定了基础。
更新日期:2020-02-20
中文翻译:

法呢基化人鸟苷酸结合蛋白1形成聚合物的分子机理。
人鸟苷酸结合蛋白1(hGBP1)属于dynamin超家族蛋白,代表先天免疫应答中的关键角色。hGBP1抗微生物病原体的活性及其抗增殖和抗肿瘤活性需要C端的法呢基化。法呢基化的hGBP1(hGBP1fn)保留了广泛研究的非法呢基化蛋白的许多特征,并获得了额外的功能,例如与脂质膜结合和hGBP1fn聚合物的形成。据信这些聚合物可作为蛋白质贮库,使该酶可立即用于抵抗细胞内病原体的入侵。在这里,我们研究hGBP1聚合物形成的分子机制,因为它是该酶的关键状态,可以实现生物学功能所需的快速响应。我们使用福斯特共振能量转移来追踪蛋白质结构域内和分子间的距离变化。光散射技术可深入了解尺寸和形状的变化。在封闭的法呢基部分隐藏状态和开放的法呢基部分暴露状态之间,由GTP水解驱动的循环代表第一相,为聚合准备分子。在聚合物生长的第二阶段中,可以将打开的hGBP1分子掺入到增长的聚合物中,在该聚合物中,打开的结构得以稳定,类似于胶束中的表面活性剂分子,将法呢基部分指向疏水中心并将头基定位在外围聚合物。我们贡献了聚合物形成的分子机制,为对hGBP1功能的详细了解奠定了基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号