当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Developing Implantable Scaffolds to Enhance Neural Stem Cell Therapy for Post-Operative Glioblastoma.
Molecular Therapy ( IF 12.4 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.ymthe.2020.02.008 Kevin T Sheets 1 , Matthew G Ewend 2 , Mahsa Mohiti-Asli 3 , Stephen A Tuin 3 , Elizabeth G Loboa 4 , Karen S Aboody 5 , Shawn D Hingtgen 6
Molecular Therapy ( IF 12.4 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.ymthe.2020.02.008 Kevin T Sheets 1 , Matthew G Ewend 2 , Mahsa Mohiti-Asli 3 , Stephen A Tuin 3 , Elizabeth G Loboa 4 , Karen S Aboody 5 , Shawn D Hingtgen 6
Affiliation
|
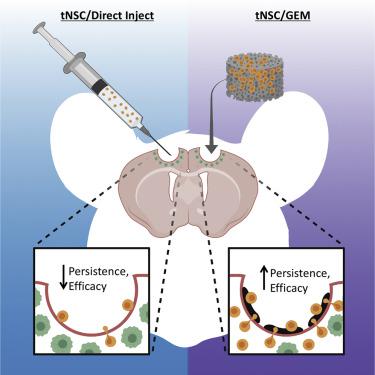
Pre-clinical and clinical studies have shown that engineered tumoricidal neural stem cells (tNSCs) are a promising treatment strategy for the aggressive brain cancer glioblastoma (GBM). Yet, stabilizing human tNSCs within the surgical cavity following GBM resection is a significant challenge. As a critical step toward advancing engineered human NSC therapy for GBM, we used a preclinical variant of the clinically utilized NSC line HB1.F3.CD and mouse models of human GBM resection/recurrence to identify a polymeric scaffold capable of maximizing the transplant, persistence, and tumor kill of NSC therapy for post-surgical GBM. Using kinetic bioluminescence imaging, we found that tNSCs delivered into the mouse surgical cavity wall by direct injection persisted only 3 days. We found that delivery of tNSCs into the cavity on nanofibrous electrospun poly-l-lactic acid scaffolds extended tNSC persistence to 8 days. Modifications to fiber surface coating, diameter, and morphology of the scaffold failed to significantly extend tNSC persistence in the cavity. In contrast, tNSCs delivered into the post-operative cavity on gelatin matrices (GEMs) persisted 8-fold longer as compared to direct injection. GEMs remained permissive to tumor-tropic homing, as tNSCs migrated off the scaffolds and into invasive tumor foci both in vitro and in vivo. To mirror envisioned human brain tumor trials, we engineered tNSCs to express the prodrug/enzyme thymidine kinase (tNSCstk) and transplanted the therapeutic cells in the post-operative cavity of mice bearing resected orthotopic patient-derived GBM xenografts. Following administration of the prodrug ganciclovir, residual tumor volumes in mice receiving GEM/tNSCs were reduced by 10-fold at day 35, and median survival was extended from 31 to 46 days. Taken together, these data begin to define design parameters for effective scaffold/tNSC composites and suggest a new approach to maximizing the efficacy of tNSC therapy in human patient trials.
中文翻译:

发展植入式支架,以增强手术后胶质母细胞瘤的神经干细胞治疗。
临床前和临床研究表明,工程杀伤性神经干细胞(tNSC)是侵略性脑癌胶质母细胞瘤(GBM)的一种有前途的治疗策略。然而,在GBM切除后稳定手术腔内的人类tNSC是一项重大挑战。作为推进针对GBM的工程人NSC治疗的关键步骤,我们使用了临床上使用的NSC HB1.F3.CD的临床前变体以及人GBM切除/复发的小鼠模型,以鉴定能够最大化移植,持久性的聚合物支架。 ,以及手术后GBM的NSC治疗对肿瘤的杀伤作用。使用动力学生物发光成像,我们发现通过直接注射递送到小鼠手术腔壁的tNSC仅持续3天。我们发现,将tNSCs递送到纳米纤维电纺丝聚乳酸支架上的空腔中可将tNSC持久性延长至8天。修改纤维表面涂层,支架的直径和形态无法显着延长tNSC在腔中的持久性。相比之下,与直接注射相比,在明胶基质(GEM)上递送至术后腔的tNSC持续时间长8倍。随着tNSCs从支架上迁移并进入体内和体外的侵袭性肿瘤灶,GEMs仍然允许肿瘤嗜性归巢。为了反映设想的人类脑肿瘤试验,我们设计了tNSCs以表达前药/酶胸苷激酶(tNSCstk),并将治疗细胞移植到携带有原位患者原位切除的GBM异种移植物的小鼠的术后腔中。给予更昔洛韦前药后,接受GEM / tNSCs的小鼠中的残留肿瘤体积在第35天减少了10倍,中位生存期从31天延长至46天。综上所述,这些数据开始为有效的脚手架/ tNSC复合材料定义设计参数,并提出了在人类患者试验中最大化tNSC治疗功效的新方法。
更新日期:2020-02-13
中文翻译:

发展植入式支架,以增强手术后胶质母细胞瘤的神经干细胞治疗。
临床前和临床研究表明,工程杀伤性神经干细胞(tNSC)是侵略性脑癌胶质母细胞瘤(GBM)的一种有前途的治疗策略。然而,在GBM切除后稳定手术腔内的人类tNSC是一项重大挑战。作为推进针对GBM的工程人NSC治疗的关键步骤,我们使用了临床上使用的NSC HB1.F3.CD的临床前变体以及人GBM切除/复发的小鼠模型,以鉴定能够最大化移植,持久性的聚合物支架。 ,以及手术后GBM的NSC治疗对肿瘤的杀伤作用。使用动力学生物发光成像,我们发现通过直接注射递送到小鼠手术腔壁的tNSC仅持续3天。我们发现,将tNSCs递送到纳米纤维电纺丝聚乳酸支架上的空腔中可将tNSC持久性延长至8天。修改纤维表面涂层,支架的直径和形态无法显着延长tNSC在腔中的持久性。相比之下,与直接注射相比,在明胶基质(GEM)上递送至术后腔的tNSC持续时间长8倍。随着tNSCs从支架上迁移并进入体内和体外的侵袭性肿瘤灶,GEMs仍然允许肿瘤嗜性归巢。为了反映设想的人类脑肿瘤试验,我们设计了tNSCs以表达前药/酶胸苷激酶(tNSCstk),并将治疗细胞移植到携带有原位患者原位切除的GBM异种移植物的小鼠的术后腔中。给予更昔洛韦前药后,接受GEM / tNSCs的小鼠中的残留肿瘤体积在第35天减少了10倍,中位生存期从31天延长至46天。综上所述,这些数据开始为有效的脚手架/ tNSC复合材料定义设计参数,并提出了在人类患者试验中最大化tNSC治疗功效的新方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号