当前位置:
X-MOL 学术
›
Kidney Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
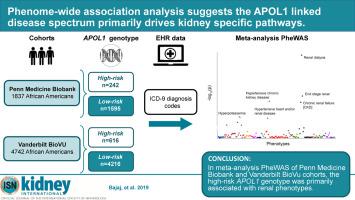
Phenome-wide association analysis suggests the APOL1 linked disease spectrum primarily drives kidney-specific pathways.
Kidney International ( IF 19.6 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.kint.2020.01.027 Archna Bajaj 1 , Andrea Ihegword 2 , Chengxiang Qiu 3 , Aeron M Small 4 , Wei-Qi Wei 5 , Lisa Bastarache 5 , QiPing Feng 2 , Rachel L Kember 6 , Marjorie Risman 1 , Roy D Bloom 3 , David L Birtwell 7 , Heather Williams 7 , Christian M Shaffer 2 , Jinbo Chen 8 , Regeneron Genetics Center 9 , Joshua C Denny 10 , Daniel J Rader 11 , C Michael Stein 12 , Scott M Damrauer 13 , Katalin Susztak 14
Kidney International ( IF 19.6 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.kint.2020.01.027 Archna Bajaj 1 , Andrea Ihegword 2 , Chengxiang Qiu 3 , Aeron M Small 4 , Wei-Qi Wei 5 , Lisa Bastarache 5 , QiPing Feng 2 , Rachel L Kember 6 , Marjorie Risman 1 , Roy D Bloom 3 , David L Birtwell 7 , Heather Williams 7 , Christian M Shaffer 2 , Jinbo Chen 8 , Regeneron Genetics Center 9 , Joshua C Denny 10 , Daniel J Rader 11 , C Michael Stein 12 , Scott M Damrauer 13 , Katalin Susztak 14
Affiliation
|
The relationship between commonly occurring genetic variants (G1 and G2) in the APOL1 gene in African Americans and different disease traits, such as kidney disease, cardiovascular disease, and pre-eclampsia, remains the subject of controversy. Here we took a genotype-first approach, a phenome-wide association study, to define the spectrum of phenotypes associated with APOL1 high-risk variants in 1,837 African American participants of Penn Medicine Biobank and 4,742 African American participants of Vanderbilt BioVU. In the Penn Medicine Biobank, outpatient creatinine measurement-based estimated glomerular filtration rate and multivariable regression models were used to evaluate the association between high-risk APOL1 status and renal outcomes. In meta-analysis of both cohorts, the strongest phenome-wide association study associations were for the high-risk APOL1 variants and diagnoses codes were highly significant for "kidney dialysis" (odds ratio 3.75) and "end stage kidney disease" (odds ratio 3.42). A number of phenotypes were associated with APOL1 high-risk genotypes in an analysis adjusted only for demographic variables. However, no associations were detected with non-renal phenotypes after controlling for chronic/end stage kidney disease status. Using calculated estimated glomerular filtration rate -based phenotype analysis in the Penn Medicine Biobank, APOL1 high-risk status was associated with prevalent chronic/end stage kidney disease /kidney transplant (odds ratio 2.27, 95% confidence interval 1.67-3.08). In high-risk participants, the estimated glomerular filtration rate was 15.4 mL/min/1.73m2; significantly lower than in low-risk participants. Thus, although APOL1 high-risk variants are associated with a range of phenotypes, the risks for other associated phenotypes appear much lower and in our dataset are driven by a primary effect on renal disease.
中文翻译:

全表型关联分析表明 APOL1 相关疾病谱主要驱动肾脏特异性途径。
非洲裔美国人 APOL1 基因中常见的遗传变异(G1 和 G2)与肾脏疾病、心血管疾病和先兆子痫等不同疾病特征之间的关系仍然存在争议。在这里,我们采用基因型优先方法,即全表型关联研究,在 Penn Medicine Biobank 的 1,837 名非裔美国人参与者和 Vanderbilt BioVU 的 4,742 名非裔美国人参与者中定义与 APOL1 高风险变异相关的表型谱。在 Penn Medicine Biobank 中,基于门诊肌酐测量的估计肾小球滤过率和多变量回归模型用于评估高危 APOL1 状态与肾脏结果之间的关联。在两个队列的荟萃分析中,最强的全表型关联研究关联是针对高风险 APOL1 变体,诊断代码对“肾透析”(优势比 3.75)和“终末期肾病”(优势比 3.42)具有高度显着性。在仅针对人口变量进行调整的分析中,许多表型与 APOL1 高风险基因型相关。然而,在控制慢性/终末期肾脏疾病状态后,未检测到与非肾脏表型的关联。使用 Penn Medicine Biobank 中计算的基于估计肾小球滤过率的表型分析,APOL1 高风险状态与流行的慢性/终末期肾病/肾移植相关(优势比 2.27,95% 置信区间 1.67-3.08)。在高风险参与者中,估计的肾小球滤过率为 15.4 mL/min/1。73平方米;显着低于低风险参与者。因此,尽管 APOL1 高风险变体与一系列表型相关,但其他相关表型的风险似乎要低得多,并且在我们的数据集中是由对肾脏疾病的主要影响驱动的。
更新日期:2020-02-17
中文翻译:

全表型关联分析表明 APOL1 相关疾病谱主要驱动肾脏特异性途径。
非洲裔美国人 APOL1 基因中常见的遗传变异(G1 和 G2)与肾脏疾病、心血管疾病和先兆子痫等不同疾病特征之间的关系仍然存在争议。在这里,我们采用基因型优先方法,即全表型关联研究,在 Penn Medicine Biobank 的 1,837 名非裔美国人参与者和 Vanderbilt BioVU 的 4,742 名非裔美国人参与者中定义与 APOL1 高风险变异相关的表型谱。在 Penn Medicine Biobank 中,基于门诊肌酐测量的估计肾小球滤过率和多变量回归模型用于评估高危 APOL1 状态与肾脏结果之间的关联。在两个队列的荟萃分析中,最强的全表型关联研究关联是针对高风险 APOL1 变体,诊断代码对“肾透析”(优势比 3.75)和“终末期肾病”(优势比 3.42)具有高度显着性。在仅针对人口变量进行调整的分析中,许多表型与 APOL1 高风险基因型相关。然而,在控制慢性/终末期肾脏疾病状态后,未检测到与非肾脏表型的关联。使用 Penn Medicine Biobank 中计算的基于估计肾小球滤过率的表型分析,APOL1 高风险状态与流行的慢性/终末期肾病/肾移植相关(优势比 2.27,95% 置信区间 1.67-3.08)。在高风险参与者中,估计的肾小球滤过率为 15.4 mL/min/1。73平方米;显着低于低风险参与者。因此,尽管 APOL1 高风险变体与一系列表型相关,但其他相关表型的风险似乎要低得多,并且在我们的数据集中是由对肾脏疾病的主要影响驱动的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号