当前位置:
X-MOL 学术
›
Kidney Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease.
Kidney International ( IF 19.6 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.kint.2020.01.028 Tessa Gryp 1 , Kim De Paepe 2 , Raymond Vanholder 3 , Frederiek-Maarten Kerckhof 2 , Wim Van Biesen 3 , Tom Van de Wiele 2 , Francis Verbeke 3 , Marijn Speeckaert 3 , Marie Joossens 4 , Marie Madeleine Couttenye 5 , Mario Vaneechoutte 6 , Griet Glorieux 3
Kidney International ( IF 19.6 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.kint.2020.01.028 Tessa Gryp 1 , Kim De Paepe 2 , Raymond Vanholder 3 , Frederiek-Maarten Kerckhof 2 , Wim Van Biesen 3 , Tom Van de Wiele 2 , Francis Verbeke 3 , Marijn Speeckaert 3 , Marie Joossens 4 , Marie Madeleine Couttenye 5 , Mario Vaneechoutte 6 , Griet Glorieux 3
Affiliation
|
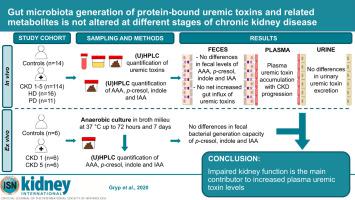
Chronic kidney disease (CKD) is characterized by accumulation of protein-bound uremic toxins such as p-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate and indole-3-acetic acid, which originate in the gut. Intestinal bacteria metabolize aromatic amino acids into p-cresol and indole, (further conjugated in the colon mucosa and liver) and indole-3-acetic acid. Here we measured fecal, plasma and urine metabolite concentrations; the contribution of gut bacterial generation to plasma protein-bound uremic toxins accumulation; and influx into the gut of circulating protein-bound uremic toxins at different stages of CKD. Feces, blood and urine were collected from 14 control individuals and 141 patients with CKD. Solutes were quantified by ultra-high performance liquid chromatography. To assess the rate of bacterial generation of p-cresol, indole and indole-3-acetic acid, fecal samples were cultured ex vivo. With CKD progression, an increase in protein-bound uremic toxins levels was observed in plasma, whereas the levels of these toxins and their precursors remained the same in feces and urine. Anaerobic culture of fecal samples showed no difference in ex vivo p-cresol, indole and indole-3-acetic acid generation. Therefore, differences in plasma protein-bound uremic toxins levels between different CKD stages cannot be explained by differences in bacterial generation rates in the gut, suggesting retention due to impaired kidney function as the main contributor to their increased plasma levels. Thus, as fractional clearance decreased with the progression of CKD, tubular clearance appeared to be more affected than the glomerular filtration rate, and there was no net increase in protein-bound uremic toxins influx into the gut lumen with increased plasma levels.
中文翻译:

在慢性肾脏病的不同阶段,与蛋白质结合的尿毒症毒素和相关代谢产物的肠道菌群的产生没有改变。
慢性肾脏疾病(CKD)的特征是蛋白质结合的尿毒症毒素的积累,例如对-甲酚硫酸盐,对-甲酚葡萄糖醛酸苷,吲哚酚硫酸盐和吲哚-3-乙酸,它们起源于肠道。肠道细菌将芳香族氨基酸代谢为对甲酚和吲哚(进一步与结肠粘膜和肝脏结合)和吲哚-3-乙酸。在这里,我们测量了粪便,血浆和尿液代谢产物的浓度。肠道细菌的产生对血浆蛋白结合的尿毒症毒素积累的贡献;并在CKD的不同阶段流入循环蛋白结合的尿毒症毒素的肠道。从14名对照个体和141名CKD患者中收集粪便,血液和尿液。通过超高效液相色谱对溶质进行定量。为了评估细菌产生对甲酚的速率,粪便样品离体培养出吲哚和吲哚-3-乙酸。随着CKD的进展,血浆中结合蛋白的尿毒症毒素水平增加,而粪便和尿液中这些毒素及其前体的水平保持不变。粪便样品的厌氧培养显示离体对甲酚,吲哚和吲哚-3-乙酸的产生没有差异。因此,不同CKD阶段之间血浆蛋白结合的尿毒症毒素水平的差异不能通过肠道细菌生成速率的差异来解释,这表明由于肾功能受损是其血浆水平升高的主要因素而导致的保留。因此,随着分数清除率随着CKD的发展而降低,肾小管清除率似乎比肾小球滤过率的影响更大,
更新日期:2020-02-17
中文翻译:

在慢性肾脏病的不同阶段,与蛋白质结合的尿毒症毒素和相关代谢产物的肠道菌群的产生没有改变。
慢性肾脏疾病(CKD)的特征是蛋白质结合的尿毒症毒素的积累,例如对-甲酚硫酸盐,对-甲酚葡萄糖醛酸苷,吲哚酚硫酸盐和吲哚-3-乙酸,它们起源于肠道。肠道细菌将芳香族氨基酸代谢为对甲酚和吲哚(进一步与结肠粘膜和肝脏结合)和吲哚-3-乙酸。在这里,我们测量了粪便,血浆和尿液代谢产物的浓度。肠道细菌的产生对血浆蛋白结合的尿毒症毒素积累的贡献;并在CKD的不同阶段流入循环蛋白结合的尿毒症毒素的肠道。从14名对照个体和141名CKD患者中收集粪便,血液和尿液。通过超高效液相色谱对溶质进行定量。为了评估细菌产生对甲酚的速率,粪便样品离体培养出吲哚和吲哚-3-乙酸。随着CKD的进展,血浆中结合蛋白的尿毒症毒素水平增加,而粪便和尿液中这些毒素及其前体的水平保持不变。粪便样品的厌氧培养显示离体对甲酚,吲哚和吲哚-3-乙酸的产生没有差异。因此,不同CKD阶段之间血浆蛋白结合的尿毒症毒素水平的差异不能通过肠道细菌生成速率的差异来解释,这表明由于肾功能受损是其血浆水平升高的主要因素而导致的保留。因此,随着分数清除率随着CKD的发展而降低,肾小管清除率似乎比肾小球滤过率的影响更大,


























 京公网安备 11010802027423号
京公网安备 11010802027423号