Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.jinorgbio.2020.111042 Gyula Tircsó , Enikő Tircsóné Benyó , Zoltán Garda , Jaspal Singh , Robert Trokowski , Ernő Brücher , A. Dean Sherry , Éva Tóth , Zoltán Kovács
|
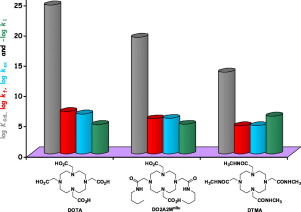
The 1,7-diacetate-4,10-diacetamide substituted 1,4,7,10-tetraazacyclododecane structural unit is common to several responsive Magnetic Resonance Imaging (MRI) contrast agents (CAs). While some of these complexes (agents capable of sensing fluctuations in Zn2+, Ca2+ etc. ions) have already been tested in vivo, the detailed physico-chemical characterization of such ligands have not been fully studied. To fill this gap, we synthesized a representative member of this ligand family possessing two acetate and two n-butylacetamide pendant side-arms (DO2A2MnBu = 1,4,7,10-tetraazacyclodoecane-1,7-di(acetic acid)-4,10-di(N-butylacetamide)), and studied its complexation properties with some essential metal and a few lanthanide(III) (Ln(III)) ions. Our studies revealed that the ligand basicity, the stability of metal ion complexes, the trend of stability constants along the Ln(III) series, the formation rates of the Ln(III) complexes and the exchange rate of the bound water molecule in the Gd(III) complex fell between those of Ln(DOTA)− and Ln(DOTA-tetra(amide))3+ complexes (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, DOTAM = 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane). The only exception is the stability of Cu(DO2A2MnBu) which was found to be only slightly lower than that of Cu(DOTA)2− (log KCuL = 19.85 vs. 21.98). This is likely reflects exclusive coordination of the negatively charged acetate donor atoms to the Cu2+ ion forming an octahedral complex with the amides remaining uncoordinated. The only anomaly observed during the study was the rates of acid assisted dissociation of the Ln(III) complexes, which occur at a rate similar to those observed for the Ln(DOTA)− complexes. These data indicate that even though the Ln(DO2A2MnBu)+ complexes have lower thermodynamic stabilities, their kinetic inertness should be sufficient for in vivo use.
中文翻译:

一些金属离子-DOTA和DOTA-双(酰胺)配合物的平衡,动力学和水交换性质的比较
1,7-二乙酸酯-4,10-二乙酰胺取代的1,4,7,10-四氮杂环十二烷结构单元是几种响应性磁共振成像(MRI)造影剂(CA)共有的。尽管其中一些复合物(能够感应Zn 2 +,Ca 2+等离子波动的试剂)已经在体内进行了测试,但尚未充分研究此类配体的详细理化特性。为了填补这一空白,我们合成了具有两个乙酸盐和两个正丁基乙酰胺侧链的侧基(DO2A2M nBu = 1,4,7,10-四氮杂环十二烷-1,7-二(乙酸)- 4,10-di(N-丁基乙酰胺)),并研究了其与一些必需金属和一些镧系元素(III)(Ln(III))离子的络合性能。我们的研究表明,配体碱性,金属离子配合物的稳定性,沿Ln(III)系列的稳定常数的趋势,Ln(III)配合物的形成速率和结合水分子在Gd中的交换速率(III)络合物介于Ln(DOTA)-和Ln(DOTA-tetra(amide))3+络合物之间(DOTA = 1,4,7,10-四氮杂环十二烷-1,4,7,10-四乙酸, DOTAM = 1,4,7,10-四(氨基甲酰基甲基)-1,4,7,10-四氮杂环十二烷)。唯一的例外是Cu(DO2A2M nBu)的稳定性,发现该稳定性仅略低于Cu(DOTA)2−(log KCuL = 19.85对21.98)。这可能反映了带负电荷的乙酸盐供体原子与Cu 2+离子的排他配位,形成了八面体配合物,而酰胺保持未配位。在研究期间观察到的唯一异常是Ln(III)配合物的酸辅助解离速率,其发生速率与Ln(DOTA)-配合物观察到的速率相似。这些数据表明,即使Ln(DO2A2M nBu)+配合物具有较低的热力学稳定性,其动力学惰性也应足以用于体内。

























 京公网安备 11010802027423号
京公网安备 11010802027423号