Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.jfluchem.2020.109493 Matthew P. Confer , Sadulla R. Allayarov , Ida P. Kim , I.V. Markin , Virgil E. Jackson , David A. Dixon
|
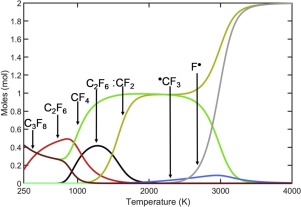
Mechanisms for the processes of direct fluorination of tetrafluoroethylene (TFE) in matrices of TFE, hexafluoropropylene (HFP), and dimers and trimers of HFP ((HFP)2 and (HFP)3) from 77 K to 300 K have been developed. Electronic structure calculations at the composite correlated G3(MP2) and G4 molecular orbital theory levels of the energetics of a range of reactions involving TFE and fluorine are presented to aid in the development of these mechanisms. The equilibrium products of the direct fluorination of TFE in the gas phase at varied temperature and initial composition was determined by Gibbs free energy minimization. Spontaneous reactions (explosions) were observed for the fluorination of pure crystalline TFE and HFP. Fluorination of TFE can be performed without explosion in glassy matrixes of (HFP)2 or (HFP)3 at low temperatures. The explosive nature of the reaction decreases in the matrix order TFE > (HFP)2 > (HFP)3. The fluorination of TFE begins at the phase transition temperature of the matrix, i.e., after the transition of devitrified (HFP)2 and (HPF)3 into the supercooled liquid state at 110 K and 150 K, respectively. The experiments show that either the presence of a branched structure (C9F20, the saturated analog of (HFP)3) or the presence of unsaturated bonds (perfluorotoluene) separately cannot provide a medium for the safe fluorination of TFE as the direct fluorination of TFE in these matrices led to an explosion. HFP oligomers provide an effective environment for TFE fluorination because of the presence of double bonds surrounded by the branched perfluorinated groups. The unsaturated bonds of the HFP oligomers are an active participant in the chemical processes involved in the safe, direct fluorination of TFE.
中文翻译:

低温下直接氟化四氟乙烯
在四氟乙烯,六氟丙烯(HFP)以及HFP((HFP)2和(HFP)3)的二聚体和三聚体基质中四氟乙烯(TFE)的直接氟化过程的机理)的开发范围从77 K到300K。提出了在涉及TFE和氟的一系列反应的高能学的复合相关G3(MP2)和G4分子轨道理论水平上的电子结构计算,以帮助开发这些机理。通过吉布斯自由能最小化确定在不同温度下气相中TFE的直接氟化和初始组成的平衡产物。观察到纯晶体TFE和HFP的氟化反应发生自发反应(爆炸)。TFE的氟化可以在低温下在(HFP)2或(HFP)3的玻璃状基质中进行而不会发生爆炸。反应的爆炸性以矩阵顺序TFE>(HFP)2 >(HFP)降低3。TFE的氟化从基质的相变温度开始,即分别在110 K和150 K的失透(HFP)2和(HPF)3转变为过冷液态之后。实验表明,存在分支结构(C 9 F 20,(HFP)3的饱和类似物)或不饱和键(全氟甲苯)的存在分别不能为TFE的安全氟化提供介质,因为TFE在这些基质中的直接氟化会导致爆炸。HFP低聚物为TFE氟化提供了有效的环境,因为存在被支链全氟化基团包围的双键。HFP低聚物的不饱和键是涉及TFE安全,直接氟化的化学过程的积极参与者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号