当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy.
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.jconrel.2020.02.025 Yang Li 1 , Jinyan Lin 1 , Zhixiong Cai 2 , Peiyuan Wang 1 , Qiang Luo 1 , Cuiping Yao 2 , Yun Zhang 3 , Zhenqing Hou 4 , Jingfeng Liu 1 , Xiaolong Liu 1
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.jconrel.2020.02.025 Yang Li 1 , Jinyan Lin 1 , Zhixiong Cai 2 , Peiyuan Wang 1 , Qiang Luo 1 , Cuiping Yao 2 , Yun Zhang 3 , Zhenqing Hou 4 , Jingfeng Liu 1 , Xiaolong Liu 1
Affiliation
|
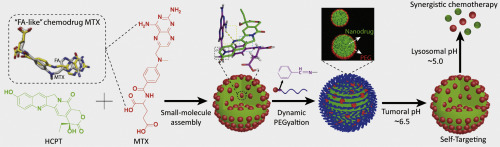
Carrier-free nanodrug via small-molecule assembly is a promising alternative strategy for tumor therapy. Thus, developing a self-recognizing carrier-free nanodrug without introduction of foreign ligand is very attractive to meet both targeting and therapeutic requirements while reducing structural complexity. Here we fabricated a tumor microenvironment-activated self-targeting nanodrug, via co-assembly of hydroxycamptothecin (HCPT) and bi-functional methotrexate (MTX, not only has antitumor effect but also shows innate affinity towards folate receptors) followed by surface covering through acidity-responsive polyethylene glycol (PEG). Notably, the morphology and size of MTX-HCPT nanodrug could be tuned by varying the drug-to-drug ratio and assembly time. The PEG shell of our nanodrug could be detached in response to acidic tumor microenvironment, and then MTX could be exposed for self-targeting to enhance tumor cell uptake. Subsequently, the shell-detached nanodrug could be dissociated in relatively stronger acidic lysosomal environment, resulting in burst release of both drugs. Further in vitro and in vivo studies demonstrated that our nanodrug showed a ~2.98-fold increase in cancer cell uptake, a ~1.25-fold increase in drug accumulation at tumor site, a significantly lower CI50 value of ~0.3, a ~27.3% improvement in tumor inhibition comparing with the corresponding non-responsive nanodrug. Taken together, the here reported tumor microenvironment-activated self-recognizing nanodrug might be an extremely promising strategy for synergistically enhancing chemotherapy efficiency with minimized side effects.
中文翻译:

肿瘤微环境激活的自我识别纳米药物通过直接定制的小分子组装进行靶向协同化疗。
通过小分子组装的无载体纳米药物是一种有前景的肿瘤治疗替代策略。因此,在不降低结构复杂性的同时,开发出无需引入外来配体的可自我识别的无载体纳米药物对于满足靶向和治疗要求非常有吸引力。在这里,我们通过羟基喜树碱(HCPT)和双功能甲氨蝶呤(MTX,不仅具有抗肿瘤作用,而且还表现出对叶酸受体的先天亲和力)的共组装作用,制备了一种通过微环境激活的自靶向纳米药物。响应性聚乙二醇(PEG)。值得注意的是,MTX-HCPT纳米药物的形态和大小可以通过改变药物与药物的比例和组装时间来调整。我们的纳米药物的PEG壳可响应酸性肿瘤微环境而脱落,然后可以暴露MTX进行自我靶向,以增强肿瘤细胞的摄取。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自我识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自我识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。
更新日期:2020-02-20
中文翻译:

肿瘤微环境激活的自我识别纳米药物通过直接定制的小分子组装进行靶向协同化疗。
通过小分子组装的无载体纳米药物是一种有前景的肿瘤治疗替代策略。因此,在不降低结构复杂性的同时,开发出无需引入外来配体的可自我识别的无载体纳米药物对于满足靶向和治疗要求非常有吸引力。在这里,我们通过羟基喜树碱(HCPT)和双功能甲氨蝶呤(MTX,不仅具有抗肿瘤作用,而且还表现出对叶酸受体的先天亲和力)的共组装作用,制备了一种通过微环境激活的自靶向纳米药物。响应性聚乙二醇(PEG)。值得注意的是,MTX-HCPT纳米药物的形态和大小可以通过改变药物与药物的比例和组装时间来调整。我们的纳米药物的PEG壳可响应酸性肿瘤微环境而脱落,然后可以暴露MTX进行自我靶向,以增强肿瘤细胞的摄取。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自我识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。随后,可以在相对更强的酸性溶酶体环境中将脱壳的纳米药物解离,从而导致两种药物的爆发释放。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自我识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。进一步的体外和体内研究表明,我们的纳米药物显示癌细胞摄取增加了约2.98倍,肿瘤部位的药物积累增加了约1.25倍,CI50值显着降低了约0.3,提高了约27.3%。与相应的无反应纳米药物相比在肿瘤抑制方面的优势。综上所述,本文报道的肿瘤微环境激活的自识别纳米药物可能是协同提高化疗效率且副作用最小的极有希望的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号