当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational analysis of the riboflavin-responsive ETF:QO-p.Pro456Leu variant associated with mild multiple acyl-CoA dehydrogenase deficiency.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.bbapap.2020.140393 Tânia G Lucas 1 , Bárbara J Henriques 1 , Cláudio M Gomes 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.bbapap.2020.140393 Tânia G Lucas 1 , Bárbara J Henriques 1 , Cláudio M Gomes 1
Affiliation
|
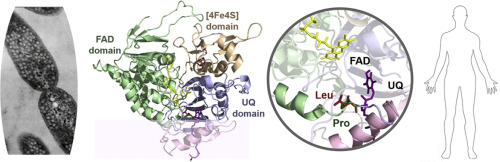
Multiple-CoA dehydrogenase deficiency (MADD) is an inborn disorder of fatty acid and amino acid metabolism caused by mutations in the genes encoding for human electron transfer flavoprotein (ETF) and its partner electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO). Albeit a rare disease, extensive newborn screening programs contributed to a wider coverage of MADD genotypes. However, the impact of non-lethal mutations on ETF:QO function remains scarcely understood from a structural perspective. To this end, we here revisit the relatively common MADD mutation ETF:QO-p.Pro456Leu, in order to clarify how it affects enzyme structure and folding. Given the limitation in recombinant expression of human ETF:QO, we resort to its bacterial homologue from Rhodobacter sphaeroides (Rs), in which the corresponding mutation (p.Pro389Leu) was inserted. The in vitro biochemical and biophysical investigations of the Rs ETF:QO-p.Pro389Leu variant showed that, while the mutation does not significantly affect the protein α/β fold, it introduces some plasticity on the tertiary structure and within flavin interactions. Indeed, in the p.Pro389Leu variant, FAD exhibits a higher thermolability during thermal denaturation and a faster rate of release in temperature-induced dissociation experiments, in comparison to the wild type. Therefore, although this clinical mutation occurs in the ubiquinone domain, its effect likely propagates to the nearby FAD binding domain, probably influencing electron transfer and redox potentials. Overall, our results provide a molecular rational for the decreased enzyme activity observed in patients and suggest that compromised FAD interactions in ETF:QO might account for the known riboflavin responsiveness of this mutation.
中文翻译:

核黄素反应性ETF:QO-p.Pro456Leu变体的构象分析,与轻度多个酰基CoA脱氢酶缺乏症相关。
多重CoA脱氢酶缺乏症(MADD)是一种先天性的脂肪酸和氨基酸代谢紊乱,由人类电子转移黄素蛋白(ETF)及其伴侣电子转移黄素蛋白:泛醌氧化还原酶(ETF:QO)的编码基因突变引起。尽管是一种罕见疾病,但广泛的新生儿筛查计划有助于MADD基因型的广泛覆盖。但是,从结构的角度来看,几乎没有了解非致命突变对ETF:QO功能的影响。为此,我们在这里重新介绍相对常见的MADD突变ETF:QO-p.Pro456Leu,以阐明其如何影响酶的结构和折叠。鉴于人类ETF:QO重组表达的局限性,我们诉诸于球形球形红细菌(Rs)的细菌同源物,其中存在相应的突变(p。Pro389Leu)已插入。Rs ETF:QO-p.Pro389Leu变体的体外生化和生物物理研究表明,尽管该突变不会显着影响蛋白质的α/β折叠,但会在三级结构和黄素相互作用中引入一些可塑性。实际上,在p.Pro389Leu变体中,与野生型相比,FAD在热变性过程中表现出更高的可热化性,并且在温度诱导的解离实验中表现出更快的释放速率。因此,尽管该临床突变发生在泛醌结构域中,但其作用可能会传播到附近的FAD结合结构域,可能影响电子转移和氧化还原电势。总体而言,我们的结果为患者体内观察到的酶活性降低提供了分子合理性,并表明ETF中FAD相互作用受到损害:
更新日期:2020-03-19
中文翻译:

核黄素反应性ETF:QO-p.Pro456Leu变体的构象分析,与轻度多个酰基CoA脱氢酶缺乏症相关。
多重CoA脱氢酶缺乏症(MADD)是一种先天性的脂肪酸和氨基酸代谢紊乱,由人类电子转移黄素蛋白(ETF)及其伴侣电子转移黄素蛋白:泛醌氧化还原酶(ETF:QO)的编码基因突变引起。尽管是一种罕见疾病,但广泛的新生儿筛查计划有助于MADD基因型的广泛覆盖。但是,从结构的角度来看,几乎没有了解非致命突变对ETF:QO功能的影响。为此,我们在这里重新介绍相对常见的MADD突变ETF:QO-p.Pro456Leu,以阐明其如何影响酶的结构和折叠。鉴于人类ETF:QO重组表达的局限性,我们诉诸于球形球形红细菌(Rs)的细菌同源物,其中存在相应的突变(p。Pro389Leu)已插入。Rs ETF:QO-p.Pro389Leu变体的体外生化和生物物理研究表明,尽管该突变不会显着影响蛋白质的α/β折叠,但会在三级结构和黄素相互作用中引入一些可塑性。实际上,在p.Pro389Leu变体中,与野生型相比,FAD在热变性过程中表现出更高的可热化性,并且在温度诱导的解离实验中表现出更快的释放速率。因此,尽管该临床突变发生在泛醌结构域中,但其作用可能会传播到附近的FAD结合结构域,可能影响电子转移和氧化还原电势。总体而言,我们的结果为患者体内观察到的酶活性降低提供了分子合理性,并表明ETF中FAD相互作用受到损害:



























 京公网安备 11010802027423号
京公网安备 11010802027423号