Journal of Photochemistry and Photobiology B: Biology ( IF 5.4 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jphotobiol.2020.111825 Sourav Das , Zaved Hazarika , Sharat Sarmah , Kakali Baruah , Mostofa Ataur Rohman , Debojit Paul , Anupam Nath Jha , Atanu Singha Roy
|
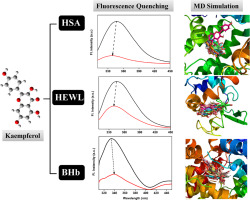
In recent years research based on kaempferol (KMP) has shown its potential therapeutic applications in medicinal chemistry and clinical biology. Therefore, to understand its molecular recognition mechanism, we studied its interactions with the carrier proteins, namely, human serum albumin (HSA), bovine hemoglobin (BHb) and hen egg white lysozyme (HEWL). The ligand, KMP was able to quench the intrinsic fluorescence of these three proteins efficiently through static quenching mode. The binding constant (Kb) for the interactions of KMP with these three proteins were found in the following order: HSA-KMP > BHb-KMP > HEWL-KMP. Different non-covalent forces such as hydrogen bonding and hydrophobic forces played a major role in the binding of KMP with HSA and HEWL, whereas hydrogen bonding and van der Waals forces contribute to the complexation of BHb with KMP. KMP was able to alter the micro-environment near the Trp fluorophore of the proteins. KMP altered the secondary structural component of all three proteins. The putative binding sites and the residues surrounding the KMP molecule within the respective protein matrix were determined through molecular docking and molecular dynamics (MD) simulation studies. The conformational flexibility of the ligand KMP and the three individual proteins were also evident from the MD simulation studies.
中文翻译:

探索生物活性山ka酚与血清白蛋白,溶菌酶和血红蛋白的相互作用:使用多光谱,对接和分子动力学模拟研究的生物物理研究
近年来,基于kaempferol(KMP)的研究显示了其在药物化学和临床生物学中的潜在治疗应用。因此,为了解其分子识别机制,我们研究了其与载体蛋白(人血清白蛋白(HSA),牛血红蛋白(BHb)和鸡蛋清溶菌酶(HEWL))的相互作用。配体KMP能够通过静态猝灭模式有效地猝灭这三种蛋白质的固有荧光。结合常数(K b),发现KMP与这三种蛋白的相互作用按以下顺序进行:HSA-KMP> BHb-KMP> HEWL-KMP。氢键和疏水力等不同的非共价力在KMP与HSA和HEWL的结合中起主要作用,而氢键和范德华力则有助于BHb与KMP的络合。KMP能够改变蛋白质Trp荧光团附近的微环境。KMP改变了所有三种蛋白质的二级结构成分。通过分子对接和分子动力学(MD)模拟研究确定了各自蛋白质基质中KMP分子周围的推定结合位点和残基。配体KMP和三种单独蛋白质的构象柔性也从MD模拟研究中明显看出。

























 京公网安备 11010802027423号
京公网安备 11010802027423号