Electrochemistry Communications ( IF 5.4 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.elecom.2020.106691 Thaze Veetil Vineesh , Valeria Yarmiayev , David Zitoun
|
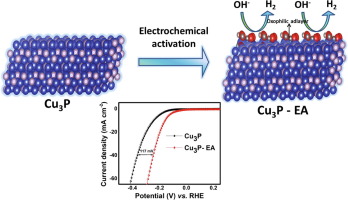
The hydrogen evolution reaction in alkaline medium can be promoted by the modification of platinum group metal with oxophilic metals. Herein, an earth abundant metal was first converted to copper phosphide Cu3P and the latter was electrochemically activated by a short and simple procedure to yield an oxidized electroactive surface. Compared with the pristine Cu3P catalyst, the electrochemical activated Cu3P catalyst exhibits an HER overpotential of 155 mV at 10 mA/cm2 (90 mV decrease from Cu3P catalyst) in alkaline medium with a stability over 24 hours). XRD, XPS and EDS reveal that the oxidation occurs only on the surface of Cu3P which shows the presence of both copper phosphide and oxide species.
中文翻译:

通过亲氧表面修饰调控Cu 3 P的电化学析氢活性。
碱性介质中的氢释放反应可通过将铂族金属与嗜氧性金属改性来促进。在此,首先将富含稀土的金属转化为磷化铜Cu 3 P,并通过短而简单的方法对其进行电化学活化,以产生氧化的电活性表面。与原始Cu 3 P催化剂相比,电化学活化的Cu 3 P催化剂在碱性介质中在10 mA / cm 2下显示出155 mV的HER过电势(比Cu 3 P催化剂降低了90 mV ),并具有24小时的稳定性。XRD,XPS和EDS表明,氧化仅发生在Cu 3的表面P表示同时存在磷化铜和氧化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号