当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and in vitro evaluation of new 5-substituted 6-nitroimidazooxazoles as antikinetoplastid agents.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-02-14 , DOI: 10.1016/j.ejmech.2020.112146 Fanny Mathias 1 , Anita Cohen 2 , Youssef Kabri 1 , Núria Waddington Negrão 3 , Maxime D Crozet 1 , Roberto Docampo 4 , Nadine Azas 2 , Patrice Vanelle 1
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-02-14 , DOI: 10.1016/j.ejmech.2020.112146 Fanny Mathias 1 , Anita Cohen 2 , Youssef Kabri 1 , Núria Waddington Negrão 3 , Maxime D Crozet 1 , Roberto Docampo 4 , Nadine Azas 2 , Patrice Vanelle 1
Affiliation
|
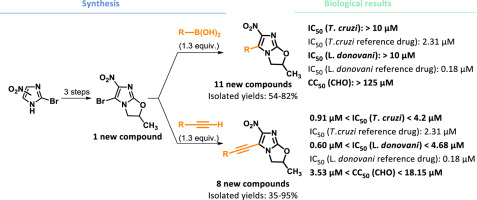
In continuation of our pharmacomodulation work on the nitroimidazooxazole series, we report the synthesis of new 5-substituted 6-nitroimidazooxazole derivatives. Our aim was to evaluate how functionalization of the 5-position of the 6-nitroimidazooxazole scaffold affects antileishmanial and antitrypanosomal in vitro activities. Twenty-one original compounds were synthesized and evaluated for their in vitro antileishmanial (L. donovani) and antitrypanosomal (T. cruzi) properties. Pallado-catalyzed cross-coupling reactions were used to introduce an aryl or ethynyl aryl substituent in 5-position from a 5-brominated-6-nitroimidazooxazole starting product. Unfortunately, the first series of compounds bearing an aryl group in 5-position presented limited in vitro activities against L. donovani and T. cruzi, with IC50 > 10 μM (vs 0.18 μM and 2.31 μM for the reference drugs amphotericin B and benznidazole respectively). Interestingly, the second series of compounds bearing an ethynyl aryl substituent in 5-position showed more promising, particularly against T. cruzi. Compounds 6a, 6b, 6c, 6g and 6h had better activity than the reference drug benznidazole (0.92 μM ≤ IC50 ≤ 2.18 μM vs IC50 = 2.31 μM), whereas the non-functionalized 2-methyl-6-nitro-2,3-dihydroimidazo [2,1-b]oxazole 2 was not active against T. cruzi (IC50 > 10 μM).
中文翻译:

新型 5-取代 6-硝基咪唑并恶唑作为抗动质体药物的合成和体外评价。
在我们对 nitroimidazooxazole 系列的药物调节工作的继续中,我们报告了新的 5-取代 6-nitroimidazooxazole 衍生物的合成。我们的目的是评估 6-硝基咪唑并恶唑支架的 5 位功能化如何影响抗利什曼原虫和抗锥虫体外活性。合成了 21 种原始化合物,并评估了它们的体外抗利什曼原虫 (L. donovani) 和抗锥虫 (T. cruzi) 特性。Pallado 催化的交叉偶联反应用于从 5-溴化-6-硝基咪唑恶唑起始产物的 5 位引入芳基或乙炔基芳基取代基。不幸的是,第一批在 5 位带有芳基的化合物对多氏乳杆菌和克氏锥虫的体外活性有限,IC50 > 10 μM(对比 0.18 μM 和 2. 参考药物两性霉素 B 和苯并硝唑分别为 31 μM)。有趣的是,在 5 位带有乙炔基芳基取代基的第二系列化合物显示出更有前途,特别是针对 T. cruzi。化合物 6a、6b、6c、6g 和 6h 比参考药物苯并硝唑具有更好的活性(0.92 μM ≤ IC50 ≤ 2.18 μM vs IC50 = 2.31 μM),而非功能化的 2-methyl-6-nitro-2,3-二氢咪唑 [2,1-b]恶唑 2 对克氏锥虫没有活性 (IC50 > 10 μM)。
更新日期:2020-02-20
中文翻译:

新型 5-取代 6-硝基咪唑并恶唑作为抗动质体药物的合成和体外评价。
在我们对 nitroimidazooxazole 系列的药物调节工作的继续中,我们报告了新的 5-取代 6-nitroimidazooxazole 衍生物的合成。我们的目的是评估 6-硝基咪唑并恶唑支架的 5 位功能化如何影响抗利什曼原虫和抗锥虫体外活性。合成了 21 种原始化合物,并评估了它们的体外抗利什曼原虫 (L. donovani) 和抗锥虫 (T. cruzi) 特性。Pallado 催化的交叉偶联反应用于从 5-溴化-6-硝基咪唑恶唑起始产物的 5 位引入芳基或乙炔基芳基取代基。不幸的是,第一批在 5 位带有芳基的化合物对多氏乳杆菌和克氏锥虫的体外活性有限,IC50 > 10 μM(对比 0.18 μM 和 2. 参考药物两性霉素 B 和苯并硝唑分别为 31 μM)。有趣的是,在 5 位带有乙炔基芳基取代基的第二系列化合物显示出更有前途,特别是针对 T. cruzi。化合物 6a、6b、6c、6g 和 6h 比参考药物苯并硝唑具有更好的活性(0.92 μM ≤ IC50 ≤ 2.18 μM vs IC50 = 2.31 μM),而非功能化的 2-methyl-6-nitro-2,3-二氢咪唑 [2,1-b]恶唑 2 对克氏锥虫没有活性 (IC50 > 10 μM)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号