Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.tet.2020.131055 Jie Zhang , Tong Zhao , Ju Xie , Zedong He , William W. Yu
|
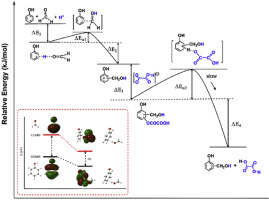
Phenolic resins are widely used as moulding plastics, coatings, industrial bondings and ablative materials. An essential reaction step for preparing phenolic resins is aromatic electrophilic substitution. However, the current understanding of this reaction mechanism is kind of too general, making them not able to explain some phenomena such as highly exothermic characteristic of the early reaction stage between phenol and aldehyde under acid catalysis, and how attacking species form when the reaction begins. We studied the detailed catalytic mechanism by quantum mechanics. A new hydrogen-bond (H-bond) catalytic mechanism is suggested. It reveals that the essence of the acid catalytic mechanism is electrophile activation through hydrogen-bonding, and the substrate-catalyst complex formed through an H-bond is the attacking species. Besides, the study discloses that oxalate anion takes part in the reaction through bonding with arenium ion. The thermodynamic and kinetic characters based on this mechanism are quite different from the current theories.
中文翻译:

苯酚和甲醛之间芳香亲电取代的H键催化机理
酚醛树脂被广泛用作模塑塑料,涂料,工业粘合剂和烧蚀材料。制备酚醛树脂的基本反应步骤是芳族亲电子取代。但是,目前对该反应机理的理解过于笼统,无法解释某些现象,例如在酸催化下苯酚和醛之间早期反应阶段的放热特性很高,以及反应开始时如何形成攻击物种。 。我们通过量子力学研究了详细的催化机理。提出了一种新的氢键(氢键)催化机理。揭示了酸催化机理的实质是通过氢键的亲电子活化,而通过氢键形成的底物-催化剂配合物是攻击物种。除了,该研究表明草酸根阴离子通过与芳烃离子键合参与反应。基于这种机理的热力学和动力学特性与目前的理论有很大不同。


























 京公网安备 11010802027423号
京公网安备 11010802027423号