当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Safety and efficacy of GABAA α5 antagonist S44819 in patients with ischaemic stroke: a multicentre, double-blind, randomised, placebo-controlled trial
The Lancet Neurology ( IF 48.0 ) Pub Date : 2020-03-01 , DOI: 10.1016/s1474-4422(20)30004-1 Hugues Chabriat 1 , Claudio L Bassetti 2 , Ute Marx 3 , Marie-Laure Audoli-Inthavong 3 , Aurore Sors 3 , Estelle Lambert 3 , Marine Wattez 3 , Dirk M Hermann 4 ,
The Lancet Neurology ( IF 48.0 ) Pub Date : 2020-03-01 , DOI: 10.1016/s1474-4422(20)30004-1 Hugues Chabriat 1 , Claudio L Bassetti 2 , Ute Marx 3 , Marie-Laure Audoli-Inthavong 3 , Aurore Sors 3 , Estelle Lambert 3 , Marine Wattez 3 , Dirk M Hermann 4 ,
Affiliation
|
BACKGROUND
S44819, a selective GABAA α5 receptor antagonist, reduces tonic post-ischaemic inhibition of the peri-infarct cortex. S44819 improved stroke recovery in rodents and increased cortical excitability in a transcranial magnetic stimulation study in healthy volunteers. The Randomized Efficacy and Safety Trial of Oral GABAA α5 antagonist S44819 after Recent ischemic Event (RESTORE BRAIN) aimed to evaluate the safety and efficacy of S44819 for enhancing clinical recovery of patients with ischaemic stroke. METHODS
RESTORE BRAIN was an international, randomised, double-blind, parallel-group, placebo-controlled, multicentre phase 2 trial that evaluated the safety and efficacy of oral S44189 in patients with recent ischaemic stroke. The study was done in specialised stroke units in 92 actively recruiting centres in 14 countries: ten were European countries (Belgium, Czech Republic, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, and the UK) and four were non-European countries (Australia, Brazil, Canada, and South Korea). Patients aged 18-85 years with acute ischaemic stroke involving cerebral cortex (National Institute of Health Stroke Scale [NIHSS] score 7-20) without previous disability were eligible for inclusion. Participants were randomly assigned to receive 150 mg S44819 twice a day, 300 mg S44819 twice a day, or placebo twice a day by a balanced, non-adaptive randomisation method with a 1:1:1 ratio. Treatment randomisation and allocation were centralised via the interactive web response system using computer-generated random sequences with a block size of 3. Blinding of treatment was achieved by identical appearance and taste of all sachets. Patients, investigators and individuals involved in the analysis of the trial were masked to group assignment. The primary endpoint was the modified Rankin Scale (mRS) score 90 days from onset of treatment, evaluated by shift analysis (predefined main analysis) or by dichotomised analyses using 0-1 versus 2-6 and 0-2 versus 3-6 cutoffs (predefined secondary analysis). Secondary endpoints were the effects of S44819 on the NIHSS and Montreal Cognitive Assessment (MoCA) scores, time needed to complete parts A and B of the Trail Making Test, and the Barthel index. Efficacy analyses were done on all patients who received at least one dose of treatment and had at least one mRS score taken after day 5 (specifically, on or after day 30). Safety was compared across treatment groups for all patients who received at least one dose of treatment. The study was registered at ClinicalTrials.gov, NCT02877615. FINDINGS
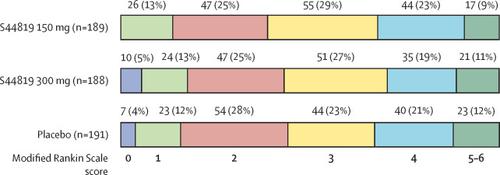
Between Dec 19, 2016, and Nov 16, 2018, 585 patients were enrolled in the study. Of these, 197 (34%) were randomly assigned to receive 150 mg S44819 twice a day, 195 (33%) to receive 300 mg S44819 twice a day, and 193 (33%) to receive placebo twice a day. 189 (96%) of 197 patients in the 150 mg S44819 group, 188 (96%) of 195 patients in the 300 mg S44819 group, and 191 (99%) patients in the placebo group received at least one dose of treatment and had at least one mRS score taken after day 5, and were included in efficacy analyses. 195 (99%) of 197 patients in the 150 mg S44819 group, 194 (99%) of 195 patients in the 300 mg S44819 group, and 193 (100%) patients in the placebo group received at least one dose of treatment, and were included in safety analyses. The primary endpoint of mRS at day 90 did not differ between each of the two S44819 groups and the placebo group (OR 0·91 [95% CI 0·64-1·31]; p=0·80 for 150 mg S44819 compared with placebo and OR 1·17 [95% CI 0·81-1·67]; p=0·80 for 300 mg S44819 compared with placebo). Likewise, dichotomised mRS scores at day 90 (mRS 0-2 vs 3-6 or mRS 0-1 vs 2-6) did not differ between groups. Secondary endpoints did not reveal any significant group differences. The median NIHSS score at day 90 did not differ between groups (4 [IQR 2-8] in 150 mg S44819 group, 4 [2-7] in 300 mg S44819 group, and 4 [2-6] in placebo group), nor did the number of patients at day 90 with an NIHSS score of up to 5 (95 [61%] of 156 in 150 mg S44819 group, 106 [66%] of 161 in 300 mg S44819 group, and 104 [66%] of 157 in placebo group) versus more than 5 (61 [39%] in 150 mg S44819 group, 55 [34%] in 300 mg S44819 group, and 53 [34%] in placebo group). Likewise, the median MoCA score (22·0 [IQR 17·0-26·0] in 150 mg S44819 group, 23·0 [19·0-26·5] in 300 mg S44819 group, and 22·0 [17·0-26·0] in placebo group), time needed to complete parts A (50 s [IQR 42-68] in 150 mg S44819 group, 49 s [36-63] in 300 mg S44819 group, and 50 s [38-68] in placebo group) and B (107 s [81-144] in 150 mg S44819 group, 121 s [76-159] in 300 mg S44819 group, and 130 s [86-175] in placebo group) of the Trail Making Test, and the Barthel index (90 [IQR 60-100] in 150 mg S44819 group, 90 [70-100] in 300 mg S44819 group, and 90 [70-100] in placebo group) were similar in all groups. Number and type of adverse events were similar between the three groups. There were no drug-related adverse events and no drug-related deaths. INTERPRETATION
There was no evidence that S44819 improved clinical outcome in patients after ischaemic stroke, and thus S44819 cannot be recommended for stroke therapy. The concept of tonic inhibition after stroke should be re-evaluated in humans. FUNDING
Servier.
中文翻译:

GABAA α5 拮抗剂 S44819 在缺血性卒中患者中的安全性和有效性:一项多中心、双盲、随机、安慰剂对照试验
背景 S44819 是一种选择性 GABAA α5 受体拮抗剂,可降低梗塞周围皮层的强直性缺血后抑制。在健康志愿者的经颅磁刺激研究中,S44819 改善了啮齿动物的中风恢复并增加了皮质兴奋性。近期缺血性事件(RESTORE BRAIN)后口服 GABAA α5 拮抗剂 S44819 的随机有效性和安全性试验旨在评估 S44819 在促进缺血性卒中患者临床康复方面的安全性和有效性。方法 RESTORE BRAIN 是一项国际、随机、双盲、平行组、安慰剂对照、多中心 2 期试验,评估口服 S44189 在近期缺血性卒中患者中的安全性和有效性。该研究是在 14 个国家/地区的 92 个积极招募中心的专门卒中单位进行的:十个是欧洲国家(比利时、捷克共和国、法国、德国、匈牙利、意大利、荷兰、波兰、西班牙和英国),四个是非欧洲国家(澳大利亚、巴西、加拿大和韩国)。年龄为 18-85 岁的急性缺血性卒中累及大脑皮层(美国国立卫生研究院卒中量表 [NIHSS] 评分 7-20)且既往无残疾的患者有资格入选。参与者被随机分配接受每天两次 150 毫克 S44819、每天两次 300 毫克 S44819 或每天两次安慰剂的平衡、非适应性随机化方法,比例为 1:1:1。处理随机化和分配通过交互式网络响应系统使用计算机生成的块大小为 3 的随机序列集中进行。通过所有小袋的相同外观和味道实现处理盲化。耐心,参与试验分析的研究人员和个人不知道分组情况。主要终点是治疗开始后 90 天的改良 Rankin 量表 (mRS) 评分,通过轮班分析(预定义的主要分析)或使用 0-1 与 2-6 和 0-2 与 3-6 截止值的二分分析进行评估。预定义的二次分析)。次要终点是 S44819 对 NIHSS 和蒙特利尔认知评估 (MoCA) 分数的影响、完成小径测试 A 和 B 部分所需的时间以及 Barthel 指数。对接受至少一剂治疗且在第 5 天后(特别是在第 30 天或之后)进行了至少一项 mRS 评分的所有患者进行了疗效分析。对接受至少一剂治疗的所有患者的治疗组之间的安全性进行了比较。该研究已在 ClinicalTrials.gov 注册,NCT02877615。结果 2016 年 12 月 19 日至 2018 年 11 月 16 日期间,共有 585 名患者参加了该研究。其中,197 人 (34%) 被随机分配接受每天两次 150 毫克 S44819,195 人 (33%) 每天两次接受 300 毫克 S44819,193 人 (33%) 每天两次接受安慰剂。150 mg S44819 组 197 名患者中有 189 名 (96%)、300 mg S44819 组 195 名患者中有 188 名 (96%) 和安慰剂组 191 名 (99%) 患者接受至少一剂治疗在第 5 天后进行至少一项 mRS 评分,并纳入疗效分析。150 mg S44819 组 197 名患者中有 195 名 (99%)、300 mg S44819 组 195 名患者中有 194 名 (99%) 和安慰剂组 193 名 (100%) 患者接受至少一剂治疗,并且被纳入安全分析。两个 S44819 组和安慰剂组的第 90 天 mRS 的主要终点没有差异(OR 0·91 [95% CI 0·64-1·31];150 mg S44819 的 p=0·80与安慰剂和 OR 1·17 [95% CI 0·81-1·67];与安慰剂相比,300 mg S44819 的 p=0·80)。同样,第 90 天的二分 mRS 评分(mRS 0-2 对 3-6 或 mRS 0-1 对 2-6)在组间没有差异。次要终点未显示任何显着的组间差异。第 90 天的中位 NIHSS 评分在各组之间没有差异(150 mg S44819 组为 4 [IQR 2-8],300 mg S44819 组为 4 [2-7],安慰剂组为 4 [2-6]),第 90 天 NIHSS 评分高达 5 分的患者数量也没有(150 毫克 S44819 组 156 人中的 95 人 [61%]、300 毫克 S44819 组中 161 人中的 106 人 [66%] 和 104 人 [66%]安慰剂组 157 人)对比 5 人以上(150 毫克 S44819 组 61 [39%],300 mg S44819 组 55 [34%],安慰剂组 53 [34%])。同样,MoCA 评分中位数(150 mg S44819 组为 22·0 [IQR 17·0-26·0],300 mg S44819 组为 23·0 [19·0-26·5],以及 22·0 [17 ·0-26·0] 安慰剂组),完成 A 部分所需的时间(150 毫克 S44819 组 50 秒 [IQR 42-68],300 毫克 S44819 组 49 秒 [36-63] 和 50 秒 [ 38-68] 安慰剂组)和 B(150 毫克 S44819 组 107 秒 [81-144],300 毫克 S44819 组 121 秒 [76-159],安慰剂组 130 秒 [86-175]) Trail Making Test 和 Barthel 指数(150 mg S44819 组为 90 [IQR 60-100],300 mg S44819 组为 90 [70-100],安慰剂组为 90 [70-100])在所有方面都相似组。三组之间不良事件的数量和类型相似。没有与药物相关的不良事件,也没有与药物相关的死亡。解释 没有证据表明 S44819 可改善缺血性卒中患者的临床结局,因此不能推荐 S44819 用于卒中治疗。应该在人类中重新评估中风后强直抑制的概念。资金服务商。
更新日期:2020-03-01
中文翻译:

GABAA α5 拮抗剂 S44819 在缺血性卒中患者中的安全性和有效性:一项多中心、双盲、随机、安慰剂对照试验
背景 S44819 是一种选择性 GABAA α5 受体拮抗剂,可降低梗塞周围皮层的强直性缺血后抑制。在健康志愿者的经颅磁刺激研究中,S44819 改善了啮齿动物的中风恢复并增加了皮质兴奋性。近期缺血性事件(RESTORE BRAIN)后口服 GABAA α5 拮抗剂 S44819 的随机有效性和安全性试验旨在评估 S44819 在促进缺血性卒中患者临床康复方面的安全性和有效性。方法 RESTORE BRAIN 是一项国际、随机、双盲、平行组、安慰剂对照、多中心 2 期试验,评估口服 S44189 在近期缺血性卒中患者中的安全性和有效性。该研究是在 14 个国家/地区的 92 个积极招募中心的专门卒中单位进行的:十个是欧洲国家(比利时、捷克共和国、法国、德国、匈牙利、意大利、荷兰、波兰、西班牙和英国),四个是非欧洲国家(澳大利亚、巴西、加拿大和韩国)。年龄为 18-85 岁的急性缺血性卒中累及大脑皮层(美国国立卫生研究院卒中量表 [NIHSS] 评分 7-20)且既往无残疾的患者有资格入选。参与者被随机分配接受每天两次 150 毫克 S44819、每天两次 300 毫克 S44819 或每天两次安慰剂的平衡、非适应性随机化方法,比例为 1:1:1。处理随机化和分配通过交互式网络响应系统使用计算机生成的块大小为 3 的随机序列集中进行。通过所有小袋的相同外观和味道实现处理盲化。耐心,参与试验分析的研究人员和个人不知道分组情况。主要终点是治疗开始后 90 天的改良 Rankin 量表 (mRS) 评分,通过轮班分析(预定义的主要分析)或使用 0-1 与 2-6 和 0-2 与 3-6 截止值的二分分析进行评估。预定义的二次分析)。次要终点是 S44819 对 NIHSS 和蒙特利尔认知评估 (MoCA) 分数的影响、完成小径测试 A 和 B 部分所需的时间以及 Barthel 指数。对接受至少一剂治疗且在第 5 天后(特别是在第 30 天或之后)进行了至少一项 mRS 评分的所有患者进行了疗效分析。对接受至少一剂治疗的所有患者的治疗组之间的安全性进行了比较。该研究已在 ClinicalTrials.gov 注册,NCT02877615。结果 2016 年 12 月 19 日至 2018 年 11 月 16 日期间,共有 585 名患者参加了该研究。其中,197 人 (34%) 被随机分配接受每天两次 150 毫克 S44819,195 人 (33%) 每天两次接受 300 毫克 S44819,193 人 (33%) 每天两次接受安慰剂。150 mg S44819 组 197 名患者中有 189 名 (96%)、300 mg S44819 组 195 名患者中有 188 名 (96%) 和安慰剂组 191 名 (99%) 患者接受至少一剂治疗在第 5 天后进行至少一项 mRS 评分,并纳入疗效分析。150 mg S44819 组 197 名患者中有 195 名 (99%)、300 mg S44819 组 195 名患者中有 194 名 (99%) 和安慰剂组 193 名 (100%) 患者接受至少一剂治疗,并且被纳入安全分析。两个 S44819 组和安慰剂组的第 90 天 mRS 的主要终点没有差异(OR 0·91 [95% CI 0·64-1·31];150 mg S44819 的 p=0·80与安慰剂和 OR 1·17 [95% CI 0·81-1·67];与安慰剂相比,300 mg S44819 的 p=0·80)。同样,第 90 天的二分 mRS 评分(mRS 0-2 对 3-6 或 mRS 0-1 对 2-6)在组间没有差异。次要终点未显示任何显着的组间差异。第 90 天的中位 NIHSS 评分在各组之间没有差异(150 mg S44819 组为 4 [IQR 2-8],300 mg S44819 组为 4 [2-7],安慰剂组为 4 [2-6]),第 90 天 NIHSS 评分高达 5 分的患者数量也没有(150 毫克 S44819 组 156 人中的 95 人 [61%]、300 毫克 S44819 组中 161 人中的 106 人 [66%] 和 104 人 [66%]安慰剂组 157 人)对比 5 人以上(150 毫克 S44819 组 61 [39%],300 mg S44819 组 55 [34%],安慰剂组 53 [34%])。同样,MoCA 评分中位数(150 mg S44819 组为 22·0 [IQR 17·0-26·0],300 mg S44819 组为 23·0 [19·0-26·5],以及 22·0 [17 ·0-26·0] 安慰剂组),完成 A 部分所需的时间(150 毫克 S44819 组 50 秒 [IQR 42-68],300 毫克 S44819 组 49 秒 [36-63] 和 50 秒 [ 38-68] 安慰剂组)和 B(150 毫克 S44819 组 107 秒 [81-144],300 毫克 S44819 组 121 秒 [76-159],安慰剂组 130 秒 [86-175]) Trail Making Test 和 Barthel 指数(150 mg S44819 组为 90 [IQR 60-100],300 mg S44819 组为 90 [70-100],安慰剂组为 90 [70-100])在所有方面都相似组。三组之间不良事件的数量和类型相似。没有与药物相关的不良事件,也没有与药物相关的死亡。解释 没有证据表明 S44819 可改善缺血性卒中患者的临床结局,因此不能推荐 S44819 用于卒中治疗。应该在人类中重新评估中风后强直抑制的概念。资金服务商。



























 京公网安备 11010802027423号
京公网安备 11010802027423号