当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Active-targeting and acid-sensitive pluronic prodrug micelles for efficiently overcoming MDR in breast cancer
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020/02/19 , DOI: 10.1039/c9tb02328c Cheng Xu 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5 , Yan Zheng 1, 2, 3, 4, 5 , Qin Fang 1, 2, 3, 4, 5 , Xiaodong Lv 1, 2, 3, 4, 5 , Xin Wang 1, 2, 3, 4, 5 , Rupei Tang 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020/02/19 , DOI: 10.1039/c9tb02328c Cheng Xu 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5 , Yan Zheng 1, 2, 3, 4, 5 , Qin Fang 1, 2, 3, 4, 5 , Xiaodong Lv 1, 2, 3, 4, 5 , Xin Wang 1, 2, 3, 4, 5 , Rupei Tang 1, 2, 3, 4, 5
Affiliation
|
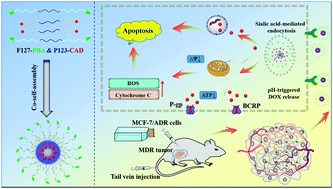
Multidrug resistance (MDR) seriously hinders therapeutic efficacy in clinical cancer treatment. Herein, we reported new polymeric prodrug micelles with tumor-targeting and acid-sensitivity properties based on two different pluronic copolymers (F127 and P123) for enhancing tumor MDR reversal and chemotherapy efficiency in breast cancer. Hybrid micelles were composed of phenylboric acid (PBA)-modified F127 (active-targeting group) and doxorubicin (DOX)-grafted P123 (prodrug groups), which were named as FBP-CAD. FBP-CAD exhibited good stability in a neutral environment and accelerated drug release under mildly acidic conditions by the cleavage of β-carboxylic amides bonds. In vitro studies demonstrated that FBP-CAD significantly increased cellular uptake and drug concentration in MCF-7/ADR cells through the homing ability of PBA and the anti-MDR effect of P123. In vivo testing further indicated that hybrid micelles facilitated drug accumulation at tumor sites as well as reduced side effects to normal organs. The synergistic effect of active-targeting and MDR-reversal leads to the highest tumor growth inhibition (TGI 78.2%). Thus, these multifunctional micelles provide a feasible approach in nanomedicine for resistant-cancer treatment.
中文翻译:

主动靶向和酸敏感的普鲁尼克前药胶束,可有效克服乳腺癌中的MDR
多药耐药性(MDR)严重阻碍了临床癌症治疗的疗效。在这里,我们报道了基于两种不同的普朗尼克共聚物(F127和P123)的具有肿瘤靶向和酸敏感性特性的新型聚合物前药胶束,可增强乳腺癌的MDR逆转和化疗效率。杂化胶束由苯硼酸(PBA)修饰的F127(活性靶向组)和阿霉素(DOX)接枝的P123(前药组)组成,它们被称为FBP-CAD。FBP-CAD在中性环境下表现出良好的稳定性,并在中等酸性条件下通过裂解β-羧基酰胺键加速药物释放。体外研究表明,FBP-CAD通过PBA的归巢能力和P123的抗MDR效应显着提高了MCF-7 / ADR细胞的细胞摄取和药物浓度。体内测试进一步表明,杂化胶束促进了药物在肿瘤部位的蓄积,并减少了对正常器官的副作用。主动靶向和MDR逆转的协同作用导致最高的肿瘤生长抑制(TGI 78.2%)。因此,这些多功能胶束为纳米药物的耐药性治疗提供了一种可行的方法。
更新日期:2020-04-01
中文翻译:

主动靶向和酸敏感的普鲁尼克前药胶束,可有效克服乳腺癌中的MDR
多药耐药性(MDR)严重阻碍了临床癌症治疗的疗效。在这里,我们报道了基于两种不同的普朗尼克共聚物(F127和P123)的具有肿瘤靶向和酸敏感性特性的新型聚合物前药胶束,可增强乳腺癌的MDR逆转和化疗效率。杂化胶束由苯硼酸(PBA)修饰的F127(活性靶向组)和阿霉素(DOX)接枝的P123(前药组)组成,它们被称为FBP-CAD。FBP-CAD在中性环境下表现出良好的稳定性,并在中等酸性条件下通过裂解β-羧基酰胺键加速药物释放。体外研究表明,FBP-CAD通过PBA的归巢能力和P123的抗MDR效应显着提高了MCF-7 / ADR细胞的细胞摄取和药物浓度。体内测试进一步表明,杂化胶束促进了药物在肿瘤部位的蓄积,并减少了对正常器官的副作用。主动靶向和MDR逆转的协同作用导致最高的肿瘤生长抑制(TGI 78.2%)。因此,这些多功能胶束为纳米药物的耐药性治疗提供了一种可行的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号