当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
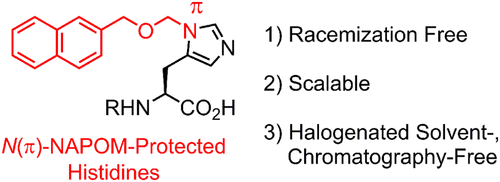
N(π)-2-Naphthylmethoxymethyl-Protected Histidines: Scalable, Racemization-Free Building Blocks for Peptide Synthesis
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.oprd.9b00538 Kohei Torikai 1 , Ryota Yanagimoto 1 , Louis A. Watanabe 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.oprd.9b00538 Kohei Torikai 1 , Ryota Yanagimoto 1 , Louis A. Watanabe 2
Affiliation
|
Histidine (His) racemizes with relative ease during peptide synthesis. One strategy to suppress this racemization is to protect the nitrogen atom of the imidazole moiety in His with a suitable protecting group. Among the numerous protecting groups that have already been tested, the p-methoxybenzyloxymethyl (PMBOM) group on the π-nitrogen atom effectively suppresses the racemization. However, a large-scale synthesis of N(π)-PMBOM-protected derivatives has hitherto been hampered by the requirement of a freshly prepared unstable reagent. Herein we report the synthesis of N(π)-2-naphthylmethoxymethyl (NAPOM)-protected His derivatives, which can be prepared on a gram scale and do not suffer from the aforementioned instability problems. Furthermore, these NAPOM-protected His derivatives suppress the racemization in Boc- and Fmoc-based peptide synthesis.
中文翻译:

N(π)-2-萘甲氧基甲基保护的组氨酸:肽合成的可扩展,无消旋结构单元
组氨酸(His)在肽合成过程中相对容易消旋。抑制该消旋作用的一种策略是用合适的保护基保护His中咪唑部分的氮原子。在已经测试的众多保护基团中,π-氮原子上的对甲氧基苄氧基甲基(PMBOM)基团有效地抑制了消旋作用。然而,迄今为止,由于需要新鲜制备的不稳定试剂,阻碍了N(π)-PMBOM-保护的衍生物的大规模合成。在此我们报告N的合成(π)-2-萘甲氧基甲基(NAPOM)-保护的His衍生物,其可以克量级制备并且不遭受上述不稳定性问题。此外,这些NAPOM保护的His衍生物可抑制基于Boc和Fmoc的肽合成中的外消旋作用。
更新日期:2020-02-26
中文翻译:

N(π)-2-萘甲氧基甲基保护的组氨酸:肽合成的可扩展,无消旋结构单元
组氨酸(His)在肽合成过程中相对容易消旋。抑制该消旋作用的一种策略是用合适的保护基保护His中咪唑部分的氮原子。在已经测试的众多保护基团中,π-氮原子上的对甲氧基苄氧基甲基(PMBOM)基团有效地抑制了消旋作用。然而,迄今为止,由于需要新鲜制备的不稳定试剂,阻碍了N(π)-PMBOM-保护的衍生物的大规模合成。在此我们报告N的合成(π)-2-萘甲氧基甲基(NAPOM)-保护的His衍生物,其可以克量级制备并且不遭受上述不稳定性问题。此外,这些NAPOM保护的His衍生物可抑制基于Boc和Fmoc的肽合成中的外消旋作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号