当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
LSD1 contributes to programmed oocyte death by regulating the transcription of autophagy adaptor SQSTM1/p62.
Aging Cell ( IF 7.8 ) Pub Date : 2020-02-19 , DOI: 10.1111/acel.13102 Meina He 1 , Tuo Zhang 1 , Zijian Zhu 1 , Shaogang Qin 1 , Huarong Wang 1 , Lihua Zhao 1 , Xinran Zhang 1 , Jiayi Hu 1 , Jia Wen 1 , Han Cai 1 , Qiliang Xin 1 , Qirui Guo 1 , Lin Lin 1 , Bo Zhou 1 , Hua Zhang 1 , Guoliang Xia 1, 2 , Chao Wang 1
Aging Cell ( IF 7.8 ) Pub Date : 2020-02-19 , DOI: 10.1111/acel.13102 Meina He 1 , Tuo Zhang 1 , Zijian Zhu 1 , Shaogang Qin 1 , Huarong Wang 1 , Lihua Zhao 1 , Xinran Zhang 1 , Jiayi Hu 1 , Jia Wen 1 , Han Cai 1 , Qiliang Xin 1 , Qirui Guo 1 , Lin Lin 1 , Bo Zhou 1 , Hua Zhang 1 , Guoliang Xia 1, 2 , Chao Wang 1
Affiliation
|
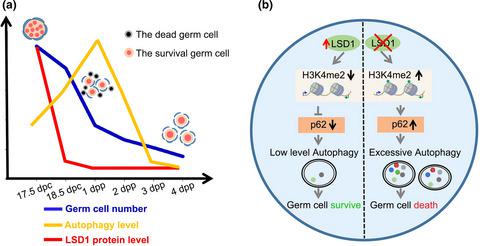
In female mammals, the size of the initially established primordial follicle (PF) pool within the ovaries determines the reproductive lifespan of females. Interestingly, the establishment of the PF pool is accompanied by a remarkable programmed oocyte loss for unclear reasons. Although apoptosis and autophagy are involved in the process of oocyte loss, the underlying mechanisms require substantial study. Here, we identify a new role of lysine‐specific demethylase 1 (LSD1) in controlling the fate of oocytes in perinatal mice through regulating the level of autophagy. Our results show that the relatively higher level of LSD1 in fetal ovaries sharply reduces from 18.5 postcoitus (dpc). Meanwhile, the level of autophagy increases while oocytes are initiating programmed death. Specific disruption of LSD1 resulted in significantly increased autophagy and obviously decreased oocyte number compared with the control. Conversely, the oocyte number is remarkably increased by the overexpression of Lsd1 in ovaries. We further demonstrated that LSD1 exerts its role by regulating the transcription of p62 and affecting autophagy level through its H3K4me2 demethylase activity. Finally, in physiological conditions, a decrease in LSD1 level leads to an increased level of autophagy in the oocyte when a large number of oocytes are being lost. Collectively, LSD1 may be one of indispensible epigenetic molecules who protects oocytes against preterm death through repressing the autophagy level in a time‐specific manner. And epigenetic modulation contributes to programmed oocyte death by regulating autophagy in mice.
中文翻译:

LSD1通过调节自噬衔接子SQSTM1 / p62的转录来促进程序性卵母细胞死亡。
在雌性哺乳动物中,卵巢内最初建立的原始卵泡(PF)库的大小决定了雌性的生殖寿命。有趣的是,由于不清楚的原因,PF池的建立伴随着明显的程序性卵母细胞丢失。尽管凋亡和自噬与卵母细胞丢失过程有关,但其潜在机制仍需要大量研究。在这里,我们确定了赖氨酸特异性脱甲基酶1(LSD1)在通过调节自噬水平来控制围生期小鼠卵母细胞命运中的新作用。我们的结果表明,胎儿卵巢中相对较高水平的LSD1从性交后(dpc)的18.5急剧降低。同时,自噬水平增加而卵母细胞开始程序性死亡。与对照组相比,LSD1的特异性破坏导致自噬显着增加,卵母细胞数目明显减少。相反,卵母细胞数由于过量表达而显着增加。卵巢中的Lsd1。我们进一步证明了LSD1通过调节p62的转录并通过其H3K4me2脱甲基酶活性影响自噬水平而发挥其作用。最后,在生理条件下,当丢失大量卵母细胞时,LSD1水平的降低会导致卵母细胞自噬水平提高。总体而言,LSD1可能是不可或缺的表观遗传分子之一,通过以特定时间的方式抑制自噬水平来保护卵母细胞免于早逝。表观遗传调节通过调节小鼠自噬而有助于程序性卵母细胞死亡。
更新日期:2020-02-19
中文翻译:

LSD1通过调节自噬衔接子SQSTM1 / p62的转录来促进程序性卵母细胞死亡。
在雌性哺乳动物中,卵巢内最初建立的原始卵泡(PF)库的大小决定了雌性的生殖寿命。有趣的是,由于不清楚的原因,PF池的建立伴随着明显的程序性卵母细胞丢失。尽管凋亡和自噬与卵母细胞丢失过程有关,但其潜在机制仍需要大量研究。在这里,我们确定了赖氨酸特异性脱甲基酶1(LSD1)在通过调节自噬水平来控制围生期小鼠卵母细胞命运中的新作用。我们的结果表明,胎儿卵巢中相对较高水平的LSD1从性交后(dpc)的18.5急剧降低。同时,自噬水平增加而卵母细胞开始程序性死亡。与对照组相比,LSD1的特异性破坏导致自噬显着增加,卵母细胞数目明显减少。相反,卵母细胞数由于过量表达而显着增加。卵巢中的Lsd1。我们进一步证明了LSD1通过调节p62的转录并通过其H3K4me2脱甲基酶活性影响自噬水平而发挥其作用。最后,在生理条件下,当丢失大量卵母细胞时,LSD1水平的降低会导致卵母细胞自噬水平提高。总体而言,LSD1可能是不可或缺的表观遗传分子之一,通过以特定时间的方式抑制自噬水平来保护卵母细胞免于早逝。表观遗传调节通过调节小鼠自噬而有助于程序性卵母细胞死亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号