Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Early treatment with FCR versus watch and wait in patients with stage Binet A high-risk chronic lymphocytic leukemia (CLL): a randomized phase 3 trial.
Leukemia ( IF 11.4 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41375-020-0747-7 Carmen D Herling 1 , Florence Cymbalista 2 , Carolin Groß-Ophoff-Müller 1 , Jasmin Bahlo 1 , Sandra Robrecht 1 , Petra Langerbeins 1 , Anna-Maria Fink 1 , Othman Al-Sawaf 1 , Raymonde Busch 3 , Raphael Porcher 4 , Bruno Cazin 5 , Brigitte Dreyfus 6 , Stefan Ibach 7 , Stéphane Leprêtre 8 , Kirsten Fischer 1 , Florian Kaiser 9 , Barbara Eichhorst 1 , Clemens-Martin Wentner 10 , Manuela A Hoechstetter 10 , Hartmut Döhner 11 , Veronique Leblond 12 , Michael Kneba 13 , Remi Letestu 2 , Sebastian Böttcher 13, 14 , Stephan Stilgenbauer 11 , Michael Hallek 1, 15 , Vincent Levy 16
Leukemia ( IF 11.4 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41375-020-0747-7 Carmen D Herling 1 , Florence Cymbalista 2 , Carolin Groß-Ophoff-Müller 1 , Jasmin Bahlo 1 , Sandra Robrecht 1 , Petra Langerbeins 1 , Anna-Maria Fink 1 , Othman Al-Sawaf 1 , Raymonde Busch 3 , Raphael Porcher 4 , Bruno Cazin 5 , Brigitte Dreyfus 6 , Stefan Ibach 7 , Stéphane Leprêtre 8 , Kirsten Fischer 1 , Florian Kaiser 9 , Barbara Eichhorst 1 , Clemens-Martin Wentner 10 , Manuela A Hoechstetter 10 , Hartmut Döhner 11 , Veronique Leblond 12 , Michael Kneba 13 , Remi Letestu 2 , Sebastian Böttcher 13, 14 , Stephan Stilgenbauer 11 , Michael Hallek 1, 15 , Vincent Levy 16
Affiliation
|
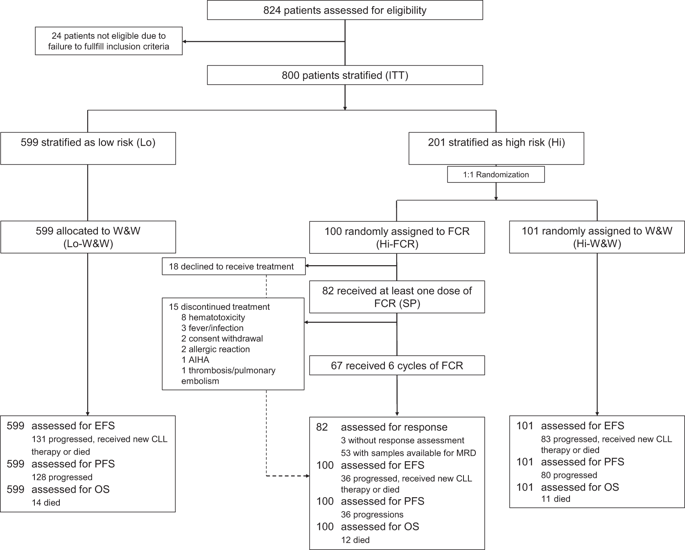
We report a randomized prospective phase 3 study (CLL7), designed to evaluate the efficacy of fludarabine, cyclophosphamide, and rituximab (FCR) in patients with an early-stage high-risk chronic lymphocytic leukemia (CLL). Eight hundred patients with untreated-stage Binet A disease were enrolled as intent-to-treat population and assessed for four prognostic markers: lymphocyte doubling time <12 months, serum thymidine kinase >10 U/L, unmutated IGHV genes, and unfavorable cytogenetics (del(11q)/del(17p)/trisomy 12). Two hundred and one patients with ≥2 risk features were classified as high-risk CLL and 1:1 randomized to receive either immediate therapy with 6xFCR (Hi-FCR, 100 patients), or to be observed according to standard of care (Hi-W&W, 101 patients). The overall response rate after early FCR was 92.7%. Common adverse events were hematological toxicities and infections (61.0%/41.5% of patients, respectively). After median observation time of 55.6 (0-99.2) months, event-free survival was significantly prolonged in Hi-FCR compared with Hi-W&W patients (median not reached vs. 18.5 months, p < 0.001). There was no significant overall survival benefit for high-risk patients receiving early FCR therapy (5-year OS 82.9% in Hi-FCR vs. 79.9% in Hi-W&W, p = 0.864). In conclusion, although FCR is efficient to induce remissions in the Binet A high-risk CLL, our data do not provide evidence that alters the current standard of care "watch and wait" for these patients.
中文翻译:

Binet分期患者的FCR早期治疗与观察和等待治疗的比较高风险的慢性淋巴细胞性白血病(CLL):一项3期随机试验。
我们报告了一项随机前瞻性3期研究(CLL7),旨在评估氟达拉滨,环磷酰胺和利妥昔单抗(FCR)在早期高危慢性淋巴细胞性白血病(CLL)患者中的疗效。将800例未经治疗的Binet A病患者纳入意向性治疗人群,并评估四个预后指标:淋巴细胞加倍时间<12个月,血清胸苷激酶> 10 U / L,IGHV基因未突变和细胞遗传学不良( del(11q)/ del(17p)/三体式12)。211位具有≥2个风险特征的患者被分类为高危CLL,并按1:1比例随机接受6xFCR立即治疗(Hi-FCR,100例患者)或根据护理标准进行观察(Hi- W&W,101位患者)。早期FCR后的总缓解率为92.7%。常见的不良事件是血液学毒性和感染(分别占患者的61.0%/ 41.5%)。在中位观察时间为55.6(0-99.2)个月后,与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。对于接受早期FCR治疗的高危患者,没有显着的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。接受早期FCR治疗的高危患者没有明显的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。接受早期FCR治疗的高危患者没有明显的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。
更新日期:2020-02-19
中文翻译:

Binet分期患者的FCR早期治疗与观察和等待治疗的比较高风险的慢性淋巴细胞性白血病(CLL):一项3期随机试验。
我们报告了一项随机前瞻性3期研究(CLL7),旨在评估氟达拉滨,环磷酰胺和利妥昔单抗(FCR)在早期高危慢性淋巴细胞性白血病(CLL)患者中的疗效。将800例未经治疗的Binet A病患者纳入意向性治疗人群,并评估四个预后指标:淋巴细胞加倍时间<12个月,血清胸苷激酶> 10 U / L,IGHV基因未突变和细胞遗传学不良( del(11q)/ del(17p)/三体式12)。211位具有≥2个风险特征的患者被分类为高危CLL,并按1:1比例随机接受6xFCR立即治疗(Hi-FCR,100例患者)或根据护理标准进行观察(Hi- W&W,101位患者)。早期FCR后的总缓解率为92.7%。常见的不良事件是血液学毒性和感染(分别占患者的61.0%/ 41.5%)。在中位观察时间为55.6(0-99.2)个月后,与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。对于接受早期FCR治疗的高危患者,没有显着的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。接受早期FCR治疗的高危患者没有明显的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。与Hi-W&W患者相比,Hi-FCR的无事件生存期显着延长(中位数未达18.5个月,p <0.001)。接受早期FCR治疗的高危患者没有明显的总体生存获益(Hi-FCR的5年OS为82.9%,而Hi-W&W为79.9%,p = 0.864)。总之,尽管FCR可以有效地缓解Binet A高危CLL的症状,但我们的数据并未提供证据改变这些患者当前的“观察和等待”护理标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号