当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of Within-Tablet Dynamics for Extended Release of a Poorly Soluble Basic Drug from Hydrophilic Matrix Tablets Using ATR-FTIR Imaging.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.molpharmaceut.9b01063 Farah Deeba Zahoor 1 , Kerstin T Mader 1 , Peter Timmins 2 , Jonathan Brown 3 , Chris Sammon 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.molpharmaceut.9b01063 Farah Deeba Zahoor 1 , Kerstin T Mader 1 , Peter Timmins 2 , Jonathan Brown 3 , Chris Sammon 1
Affiliation
|
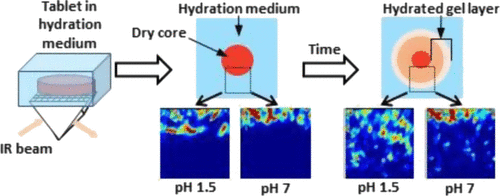
Hydrophilic matrices are an effective option for oral controlled release but can face challenges in terms of bioavailability and efficacy when used in conjunction with poorly soluble, weakly basic drugs. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) imaging provides dynamic information relating to the location and chemical nature of both the sustained release matrix and the active pharmaceutical ingredient (API) during hydration/dissolution. In this study, we have identified a model system combining itraconazole (IT), a poorly soluble, weakly basic API that has pKa in the physiological range, and hydroxypropyl methylcellulose, which is a commonly used oral tablet matrix. This system was investigated to determine the swelling kinetics at different pH values at a fixed ionic strength and to facilitate the study of the influence of hydrating media pH on the drug particle movement (translocation). Using ATR-FTIR imaging, we were able to show that gel layer formation and swelling were independent of pH but highly dependent on the ionic strength of the hydrating medium in placebo tablets. When the ionic strength was fixed, gel layer formation and radial swelling were both shown to be pH-dependent when IT was incorporated into the matrix. This was verified using optical imaging. The chemical specificity of ATR-FTIR imaging permitted the observation of transformational changes of IT from the free base to the ionized form in the tablet core during hydration. This phenomenon was shown to be greater at pH 1.5 than at pH 7. ATR-FTIR imaging was able to follow drug particle translocation at both pH 1.5 and pH 7; however, the extent of migration away from the tablet core was shown to be greater at lower pH. The location of the translocated particles within the gel layer was different between the two studied pH values, with particles being located close to the swelling front at pH 7 and within the diffusion front at pH 1.5. In both pH environments, the translocated IT particles were shown to be predominantly in the free base form. No evidence of fully solubilized IT was observed in the surrounding medium because of the inherent aqueous solubility of IT being below the instrument detection limits. This work highlighted the value of utilizing a chemically specific spectroscopic tool to increase the understanding of the nature of the factors affecting the release of a pH-dependent, poorly soluble drug from a hydrophilic matrix at different pH values and permitted greater insights into what happens inside the polymer matrix during drug release.
中文翻译:

使用ATR-FTIR成像技术从亲水性基质片剂中缓慢释放难溶性碱性药物的片内动力学研究。
亲水性基质是口服控释的有效选择,但与难溶性,弱碱性药物一起使用时,在生物利用度和功效方面可能面临挑战。衰减的全反射傅立叶变换红外(ATR-FTIR)成像可提供与水合/溶解过程中缓释基质和活性药物成分(API)的位置和化学性质有关的动态信息。在这项研究中,我们确定了一个模型系统,该模型系统结合了伊曲康唑(IT),一种在生理范围内具有pKa的难溶,弱碱性API和羟丙基甲基纤维素,后者是一种常用的口服片剂基质。研究该系统以确定在固定离子强度下在不同pH值下的溶胀动力学,并有助于研究水合介质pH对药物颗粒运动(易位)的影响。使用ATR-FTIR成像,我们能够证明凝胶层的形成和溶胀与pH无关,但高度依赖于安慰剂片剂中水合介质的离子强度。当固定离子强度时,当将IT掺入基质中时,凝胶层的形成和径向膨胀均显示为pH依赖性。使用光学成像验证了这一点。ATR-FTIR成像的化学特异性可以观察到水合期间片剂核心中IT从游离碱到离子化形式的转变变化。已显示在pH 1.5时此现象比在pH 7时更大。ATR-FTIR成像能够在pH 1.5和pH 7时跟踪药物颗粒移位;然而,在较低的pH值下,从片剂核心迁移出来的程度更大。在两个研究的pH值之间,凝胶层内易位颗粒的位置不同,颗粒在pH值为7时靠近溶胀前沿,而在pH值为1.5时位于扩散前沿。在两种pH环境中,都表明易位的IT颗粒主要呈游离碱形式。由于IT的固有水溶性低于仪器检测极限,因此在周围介质中未观察到完全溶解的IT的证据。
更新日期:2020-02-18
中文翻译:

使用ATR-FTIR成像技术从亲水性基质片剂中缓慢释放难溶性碱性药物的片内动力学研究。
亲水性基质是口服控释的有效选择,但与难溶性,弱碱性药物一起使用时,在生物利用度和功效方面可能面临挑战。衰减的全反射傅立叶变换红外(ATR-FTIR)成像可提供与水合/溶解过程中缓释基质和活性药物成分(API)的位置和化学性质有关的动态信息。在这项研究中,我们确定了一个模型系统,该模型系统结合了伊曲康唑(IT),一种在生理范围内具有pKa的难溶,弱碱性API和羟丙基甲基纤维素,后者是一种常用的口服片剂基质。研究该系统以确定在固定离子强度下在不同pH值下的溶胀动力学,并有助于研究水合介质pH对药物颗粒运动(易位)的影响。使用ATR-FTIR成像,我们能够证明凝胶层的形成和溶胀与pH无关,但高度依赖于安慰剂片剂中水合介质的离子强度。当固定离子强度时,当将IT掺入基质中时,凝胶层的形成和径向膨胀均显示为pH依赖性。使用光学成像验证了这一点。ATR-FTIR成像的化学特异性可以观察到水合期间片剂核心中IT从游离碱到离子化形式的转变变化。已显示在pH 1.5时此现象比在pH 7时更大。ATR-FTIR成像能够在pH 1.5和pH 7时跟踪药物颗粒移位;然而,在较低的pH值下,从片剂核心迁移出来的程度更大。在两个研究的pH值之间,凝胶层内易位颗粒的位置不同,颗粒在pH值为7时靠近溶胀前沿,而在pH值为1.5时位于扩散前沿。在两种pH环境中,都表明易位的IT颗粒主要呈游离碱形式。由于IT的固有水溶性低于仪器检测极限,因此在周围介质中未观察到完全溶解的IT的证据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号