当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding of Metal-Ion-Induced Tau Oligomers to Lipid Surfaces Is Enhanced by GSK-3β-Mediated Phosphorylation.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00459 Georg S Nuebling 1, 2, 3 , Eva Plesch 2 , Viktoria C Ruf 2 , Tobias Högen 1 , Stefan Lorenzl 1, 3, 4 , Frits Kamp 2, 5 , Armin Giese 2 , Johannes Levin 1, 6
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00459 Georg S Nuebling 1, 2, 3 , Eva Plesch 2 , Viktoria C Ruf 2 , Tobias Högen 1 , Stefan Lorenzl 1, 3, 4 , Frits Kamp 2, 5 , Armin Giese 2 , Johannes Levin 1, 6
Affiliation
|
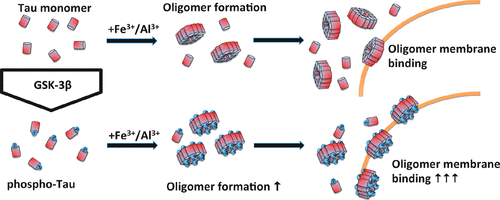
While fibrillar deposits of hyperphosphorylated protein tau are a key hallmark of several neurodegenerative diseases such as Alzheimer's disease, small oligomers have been speculated to be the key toxic aggregate species. Trivalent metal ions were shown to promote tau oligomer formation in vitro. However, little is known about potential intercellular spreading mechanisms or toxic modes of action of such oligomers. We investigated interactions of tau monomers and Fe3+/Al3+-induced oligomers with small unilamellar vesicles derived from 1-palmitoyl-2-oleoyl-phosphatidylcholine (neutral, liquid-crystalline phase) and dipalmitoyl-phosphatidylcholine (neutral, gel-phase). We further evaluated the influence of glycogen synthase kinase 3β (GSK-3β)-mediated tau phosphorylation applying the single-particle fluorescence spectroscopy techniques fluorescence correlation spectroscopy, fluorescence intensity distribution analysis, and scanning for intensely fluorescent targets. In these experiments, no binding to neutral lipid surfaces was observed for tau monomers. In contrast, metal-ion-induced tau oligomers showed a gain of function in binding to neutral lipid surfaces. Of note, tau phosphorylation by GSK-3β increased both oligomer formation and membrane affinity of the resulting oligomers. In conclusion, our data imply a pathological gain of function of metal-ion-induced oligomers of hyperphosphorylated tau, enabling membrane binding irrespective of surface charge even at nanomolar protein concentrations.
中文翻译:

GSK-3β介导的磷酸化作用增强了金属离子诱导的Tau低聚物与脂质表面的结合。
虽然高磷酸化蛋白tau的纤维状沉积物是几种神经退行性疾病(例如阿尔茨海默氏病)的关键特征,但人们推测小寡聚物是关键的毒性聚集物。已显示三价金属离子在体外可促进tau低聚物的形成。然而,对于这种寡聚体的潜在的细胞间传播机制或毒性作用方式知之甚少。我们调查了tau单体和Fe3 + / Al3 +诱导的低聚物与衍生自1-棕榈酰基-2-油酰基磷脂酰胆碱(中性,液晶相)和二棕榈酰基磷脂酰胆碱(中性,凝胶相)的单层小囊泡的相互作用。我们进一步评估了糖原合酶激酶3β(GSK-3β)介导的tau磷酸化的影响,应用了单粒子荧光光谱技术,荧光相关光谱,荧光强度分布分析和强荧光目标扫描。在这些实验中,未观察到tau单体与中性脂质表面的结合。相反,金属离子诱导的tau低聚物显示出与中性脂质表面结合的功能增强。值得注意的是,GSK-3β使tau磷酸化增加了寡聚物的形成和所得寡聚物的膜亲和力。总而言之,我们的数据暗示了金属离子诱导的高磷酸化tau寡聚物功能的病理学增强,即使在纳摩尔蛋白浓度下,无论表面电荷如何,都能够进行膜结合。
更新日期:2020-03-02
中文翻译:

GSK-3β介导的磷酸化作用增强了金属离子诱导的Tau低聚物与脂质表面的结合。
虽然高磷酸化蛋白tau的纤维状沉积物是几种神经退行性疾病(例如阿尔茨海默氏病)的关键特征,但人们推测小寡聚物是关键的毒性聚集物。已显示三价金属离子在体外可促进tau低聚物的形成。然而,对于这种寡聚体的潜在的细胞间传播机制或毒性作用方式知之甚少。我们调查了tau单体和Fe3 + / Al3 +诱导的低聚物与衍生自1-棕榈酰基-2-油酰基磷脂酰胆碱(中性,液晶相)和二棕榈酰基磷脂酰胆碱(中性,凝胶相)的单层小囊泡的相互作用。我们进一步评估了糖原合酶激酶3β(GSK-3β)介导的tau磷酸化的影响,应用了单粒子荧光光谱技术,荧光相关光谱,荧光强度分布分析和强荧光目标扫描。在这些实验中,未观察到tau单体与中性脂质表面的结合。相反,金属离子诱导的tau低聚物显示出与中性脂质表面结合的功能增强。值得注意的是,GSK-3β使tau磷酸化增加了寡聚物的形成和所得寡聚物的膜亲和力。总而言之,我们的数据暗示了金属离子诱导的高磷酸化tau寡聚物功能的病理学增强,即使在纳摩尔蛋白浓度下,无论表面电荷如何,都能够进行膜结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号