当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Insight into the Binding Profile of the Second-Generation PET Tracer PI2620 with Tau Fibrils.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00578 Guanglin Kuang 1 , N Arul Murugan 1 , Yang Zhou 1 , Agneta Nordberg 2, 3 , Hans Ågren 1, 4
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00578 Guanglin Kuang 1 , N Arul Murugan 1 , Yang Zhou 1 , Agneta Nordberg 2, 3 , Hans Ågren 1, 4
Affiliation
|
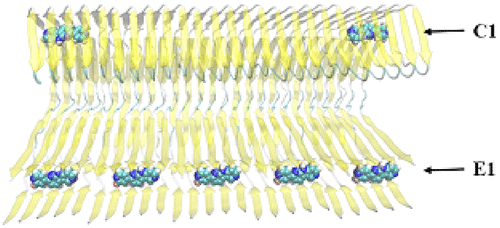
Abnormal deposition of hyperphosphorylated tau as neurofibrillary tangles (NFTs) is an important pathological hallmark of Alzheimer's disease (AD) and of other neurodegenerative disorders. A noninvasive positron emission tomography (PET) tracer that quantifies neurofibrillary tangles in vivo can enhance the clinical diagnosis of AD and can also be used to evaluate the efficacy of therapeutics aimed at reducing the abnormal aggregation of the tau fibril in the brain. In this paper, we study the binding profile of fibrillar tau aggregates with a PET tracer PI2620, which is a new second generation tau PET tracer that is presently experimentally and clinically studied. The target structure for the tau fibril is based on cryo-electron microscopy (cryo-EM) structure. A multiscale simulation workflow including molecular docking, molecular dynamics simulation, metadynamics simulation, and free energy calculations was implemented. We find that PI2620 can bind to eight surface binding sites, three core binding sites, and one entry site. The binding at the core sites and entry site is found to be much more favorable than that on the surface sites due to stronger hydrophobic interactions and less solvent exposure. Furthermore, the entry site which is formed by the terminal β-sheets of the fibril is found to have the highest binding affinity to PI2620. Importantly, the binding capacity at the entry site can be much higher than that at other core sites, due to its easy accessibility. Therefore, the entry site is believed to be the major binding site for PI2620. A previous computational study on tracers with tau fibrils reports a maximum of four binding sites. Through use of methods that allow us to locate "cryptic binding sites", we report here additional core sites available for binding and we address the limitation of using the cryo-EM structure alone for structure-based tracer design. Our results could be helpful for elucidating the binding mechanism of imaging tracers with the fibrillar form of tau, a knowledge that in turn can be used to guide the development of compounds with higher affinity and selectivity for tau using structure-based design strategies.
中文翻译:

通过计算洞察第二代PET示踪剂PI2620与Tau纤维的结合情况。
作为神经原纤维缠结(NFT)的过度磷酸化tau的异常沉积是阿尔茨海默氏病(AD)和其他神经退行性疾病的重要病理标志。定量体内神经纤维缠结的非侵入性正电子发射断层扫描(PET)示踪剂可以增强AD的临床诊断,也可以用于评估旨在减少tau纤维在大脑中异常聚集的疗法的功效。在本文中,我们用PET示踪剂PI2620研究纤维状tau聚集体的结合特性,该示踪剂是目前正在实验和临床上研究的新型第二代tau PET示踪剂。tau原纤维的靶结构基于低温电子显微镜(cryo-EM)结构。多尺度模拟工作流程,包括分子对接,实施了分子动力学模拟,元动力学模拟和自由能计算。我们发现PI2620可以结合到八个表面结合位点,三个核心结合位点和一个进入位点。由于更强的疏水相互作用和较少的溶剂暴露,发现在核心位点和进入位点的结合比在表面位点上的结合更有利。此外,发现由原纤维的末端β-片形成的进入位点对PI2620具有最高的结合亲和力。重要的是,由于易于访问,入口站点的绑定能力可能比其他核心站点的绑定能力高得多。因此,认为进入位点是PI2620的主要结合位点。先前对带有tau原纤维的示踪剂的计算研究报告最多包含四个结合位点。通过使用允许我们定位“密码结合位点”的方法,我们在此报告了可用于绑定的其他核心位点,并且我们解决了仅将cryo-EM结构用于基于结构的示踪剂设计的局限性。我们的结果可能有助于阐明成像示踪剂与tau的原纤维形式的结合机理,这一知识反过来又可以用来指导使用基于结构的设计策略开发对tau具有更高亲和力和选择性的化合物。
更新日期:2020-02-28
中文翻译:

通过计算洞察第二代PET示踪剂PI2620与Tau纤维的结合情况。
作为神经原纤维缠结(NFT)的过度磷酸化tau的异常沉积是阿尔茨海默氏病(AD)和其他神经退行性疾病的重要病理标志。定量体内神经纤维缠结的非侵入性正电子发射断层扫描(PET)示踪剂可以增强AD的临床诊断,也可以用于评估旨在减少tau纤维在大脑中异常聚集的疗法的功效。在本文中,我们用PET示踪剂PI2620研究纤维状tau聚集体的结合特性,该示踪剂是目前正在实验和临床上研究的新型第二代tau PET示踪剂。tau原纤维的靶结构基于低温电子显微镜(cryo-EM)结构。多尺度模拟工作流程,包括分子对接,实施了分子动力学模拟,元动力学模拟和自由能计算。我们发现PI2620可以结合到八个表面结合位点,三个核心结合位点和一个进入位点。由于更强的疏水相互作用和较少的溶剂暴露,发现在核心位点和进入位点的结合比在表面位点上的结合更有利。此外,发现由原纤维的末端β-片形成的进入位点对PI2620具有最高的结合亲和力。重要的是,由于易于访问,入口站点的绑定能力可能比其他核心站点的绑定能力高得多。因此,认为进入位点是PI2620的主要结合位点。先前对带有tau原纤维的示踪剂的计算研究报告最多包含四个结合位点。通过使用允许我们定位“密码结合位点”的方法,我们在此报告了可用于绑定的其他核心位点,并且我们解决了仅将cryo-EM结构用于基于结构的示踪剂设计的局限性。我们的结果可能有助于阐明成像示踪剂与tau的原纤维形式的结合机理,这一知识反过来又可以用来指导使用基于结构的设计策略开发对tau具有更高亲和力和选择性的化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号