当前位置:
X-MOL 学术
›
Oncogenesis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PDK1 promotes ovarian cancer metastasis by modulating tumor-mesothelial adhesion, invasion, and angiogenesis via α5β1 integrin and JNK/IL-8 signaling.
Oncogenesis ( IF 6.2 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41389-020-0209-0 Michelle K Y Siu 1 , Yu-Xin Jiang 1 , Jing-Jing Wang 1 , Thomas H Y Leung 1 , Siew Fei Ngu 1 , Annie N Y Cheung 2 , Hextan Y S Ngan 1 , Karen K L Chan 1
Oncogenesis ( IF 6.2 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41389-020-0209-0 Michelle K Y Siu 1 , Yu-Xin Jiang 1 , Jing-Jing Wang 1 , Thomas H Y Leung 1 , Siew Fei Ngu 1 , Annie N Y Cheung 2 , Hextan Y S Ngan 1 , Karen K L Chan 1
Affiliation
|
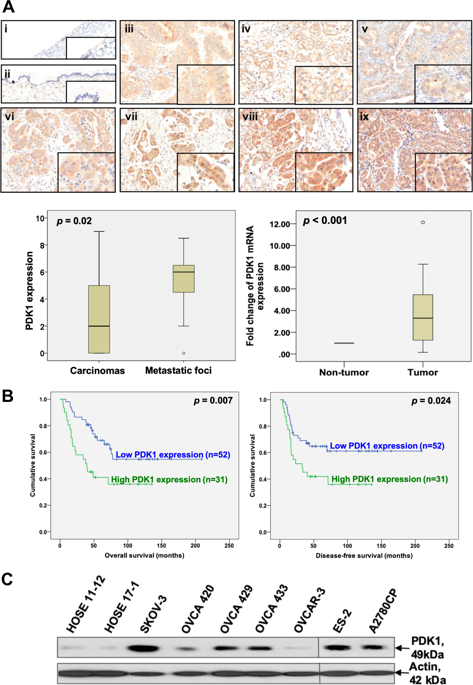
Ovarian cancer is the most lethal gynecological malignancies owing to the lack of definitive symptoms until development of widespread metastases. Identification of novel prognostic and therapeutic targets is therefore an urgent need to improve survival. Here, we demonstrated high expression of the mitochondrial gatekeeping enzyme, pyruvate dehydrogenase kinase 1 (PDK1), in both clinical samples and cell lines of ovarian cancer. PDK1 expression was significantly associated with metastasis, reduced chemosensitivity, and poor overall and disease-free survival, and further highlighted as an independent prognostic factor. Silencing of PDK1 retarded lactate production, ovarian cancer cell adhesion, migration, invasion, and angiogenesis, and consequently metastasis, concomitant with decreased α5β1 integrin expression. Phospho-kinase array profiling and RNA sequencing analyses further revealed reduction of JNK activation and IL-8 expression in PDK1-depleted cells. Conversely, PDK1 overexpression promoted cell adhesion via modulation of α5β1 integrins, along with cell migration, invasion, and angiogenesis through activation of JNK/IL-8 signaling. PDK1 depletion additionally hindered tumor growth and dissemination in nude mice in vivo. Importantly, PDK1 levels were upregulated upon treatment with conditioned medium from omental tissues, which in turn promoted metastasis. Our findings suggest that PDK1, which is regulated by the tumor microenvironment, controls lactate production and promotes ovarian cancer cell metastasis via modulation of α5β1 integrin and JNK/IL-8 signaling. To our knowledge, this is the first report to demonstrate an association between PDK1 and survival in patients with ovarian cancer, supporting its efficacy as a valuable prognostic marker and therapeutic molecular target for the disease.
中文翻译:

PDK1通过α5β1整合素和JNK / IL-8信号传导调节肿瘤-间皮的粘附,侵袭和血管生成,从而促进卵巢癌转移。
卵巢癌是最致命的妇科恶性肿瘤,原因是直到发生广泛转移之前,尚无明确的症状。因此,鉴定新的预后和治疗靶标是提高生存率的迫切需要。在这里,我们证明了卵巢癌的临床样本和细胞系中线粒体看门酶,丙酮酸脱氢酶激酶1(PDK1)的高表达。PDK1的表达与转移,化学敏感性降低以及较差的总体生存率和无疾病生存率显着相关,并进一步强调是独立的预后因素。PDK1的沉默会延迟乳酸的产生,卵巢癌细胞的粘附,迁移,侵袭和血管生成,并因此而导致转移,并伴随α5β1整联蛋白表达的降低。磷酸激酶阵列分析和RNA测序分析进一步揭示了PDK1缺失细胞中JNK激活和IL-8表达的降低。相反,PDK1过表达通过调节α5β1整合素促进细胞粘附,并通过激活JNK / IL-8信号传导促进细胞迁移,侵袭和血管生成。PDK1耗竭还阻碍了裸鼠体内肿瘤的生长和扩散。重要的是,用网膜组织的条件培养基处理后,PDK1的水平上调,从而促进了转移。我们的发现表明,受肿瘤微环境调节的PDK1通过调节α5β1整合素和JNK / IL-8信号来控制乳酸的产生并促进卵巢癌细胞的转移。据我们所知,
更新日期:2020-02-18
中文翻译:

PDK1通过α5β1整合素和JNK / IL-8信号传导调节肿瘤-间皮的粘附,侵袭和血管生成,从而促进卵巢癌转移。
卵巢癌是最致命的妇科恶性肿瘤,原因是直到发生广泛转移之前,尚无明确的症状。因此,鉴定新的预后和治疗靶标是提高生存率的迫切需要。在这里,我们证明了卵巢癌的临床样本和细胞系中线粒体看门酶,丙酮酸脱氢酶激酶1(PDK1)的高表达。PDK1的表达与转移,化学敏感性降低以及较差的总体生存率和无疾病生存率显着相关,并进一步强调是独立的预后因素。PDK1的沉默会延迟乳酸的产生,卵巢癌细胞的粘附,迁移,侵袭和血管生成,并因此而导致转移,并伴随α5β1整联蛋白表达的降低。磷酸激酶阵列分析和RNA测序分析进一步揭示了PDK1缺失细胞中JNK激活和IL-8表达的降低。相反,PDK1过表达通过调节α5β1整合素促进细胞粘附,并通过激活JNK / IL-8信号传导促进细胞迁移,侵袭和血管生成。PDK1耗竭还阻碍了裸鼠体内肿瘤的生长和扩散。重要的是,用网膜组织的条件培养基处理后,PDK1的水平上调,从而促进了转移。我们的发现表明,受肿瘤微环境调节的PDK1通过调节α5β1整合素和JNK / IL-8信号来控制乳酸的产生并促进卵巢癌细胞的转移。据我们所知,


























 京公网安备 11010802027423号
京公网安备 11010802027423号