Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Time-Domain Terahertz Spectroscopy and Density Functional Theory Studies of Nitro/Nitrogen-Rich Aryl-Tetrazole Derivatives.
ACS Omega ( IF 4.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acsomega.8b03383 Damarla Ganesh 1 , Elaprolu Narsimha Rao 1 , Mottamchetty Venkatesh 1, 2 , Kommu Nagarjuna 1 , Ganapathy Vaitheeswaran 1, 1 , Akhila K Sahoo 1, 1 , Anil K Chaudhary 1
ACS Omega ( IF 4.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acsomega.8b03383 Damarla Ganesh 1 , Elaprolu Narsimha Rao 1 , Mottamchetty Venkatesh 1, 2 , Kommu Nagarjuna 1 , Ganapathy Vaitheeswaran 1, 1 , Akhila K Sahoo 1, 1 , Anil K Chaudhary 1
Affiliation
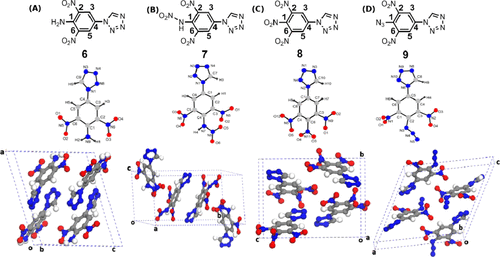
|
The paper reports the time-domain THz spectroscopy studies of noncentrosymmetric energetic nitro/nitrogen-rich aryl-tetrazole high-energy molecules. The fingerprint spectra in the THz domain reveal the role of different functional groups attached to position "1" of the tetrazole moiety, which controls the energetic properties. These responses are deliberated through density functional theory (DFT) calculations. The synthesized aryl-tetrazoles exhibit high positive heat of formation (369-744 kJ/mol), high detonation velocities, and pressures (D v: 7734-8298 m·s-1; D p: 24-28 GPa) in comparison to the noncentrosymmetric 2,4,6-trinitrotoluene (TNT). These compounds exhibit variation in the refractive indices and absorption between 0.1 and 2.2 THz range. The DFT studies at the molecular and single-crystal level (using plane wave pseudo potential method) endorse in detecting these bands (with ∼1% deviation). The calculated vibrational frequencies and linear optical properties are found to have good agreement with the experimental data in UV-visible and THz regions.
中文翻译:

氮/富氮芳基-四唑衍生物的时域太赫兹光谱学和密度泛函理论研究。
该论文报道了非中心对称高能氮/富氮芳基-四唑高能分子的时域太赫兹光谱研究。在THz域中的指纹图谱揭示了连接到四唑部分位置“ 1”的不同官能团的作用,该官能团控制着能量特性。这些响应是通过密度泛函理论(DFT)计算来审议的。与之相比,合成的芳基四唑具有较高的正形成热(369-744 kJ / mol),高爆轰速度和压力(D v:7734-8298 m·s-1; D p:24-28 GPa)非中心对称的2,4,6-三硝基甲苯(TNT)。这些化合物的折射率和吸收率在0.1至2.2 THz范围内变化。DFT在分子和单晶水平上的研究(使用平面波伪电位法)支持检测这些谱带(偏差约为1%)。发现计算出的振动频率和线性光学特性与紫外可见光和太赫兹区域的实验数据具有良好的一致性。
更新日期:2020-02-18
中文翻译:

氮/富氮芳基-四唑衍生物的时域太赫兹光谱学和密度泛函理论研究。
该论文报道了非中心对称高能氮/富氮芳基-四唑高能分子的时域太赫兹光谱研究。在THz域中的指纹图谱揭示了连接到四唑部分位置“ 1”的不同官能团的作用,该官能团控制着能量特性。这些响应是通过密度泛函理论(DFT)计算来审议的。与之相比,合成的芳基四唑具有较高的正形成热(369-744 kJ / mol),高爆轰速度和压力(D v:7734-8298 m·s-1; D p:24-28 GPa)非中心对称的2,4,6-三硝基甲苯(TNT)。这些化合物的折射率和吸收率在0.1至2.2 THz范围内变化。DFT在分子和单晶水平上的研究(使用平面波伪电位法)支持检测这些谱带(偏差约为1%)。发现计算出的振动频率和线性光学特性与紫外可见光和太赫兹区域的实验数据具有良好的一致性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号