当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stoichiometric Studies on the Carbonylative Trifluoromethylation of Aryl Pd(II) Complexes using TMSCF3 as the Trifluoromethyl Source
Organometallics ( IF 2.8 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.organomet.9b00849 Katrine Domino 1 , Martin B. Johansen 1, 2 , Kim Daasbjerg 1 , Troels Skrydstrup 1
Organometallics ( IF 2.8 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.organomet.9b00849 Katrine Domino 1 , Martin B. Johansen 1, 2 , Kim Daasbjerg 1 , Troels Skrydstrup 1
Affiliation
|
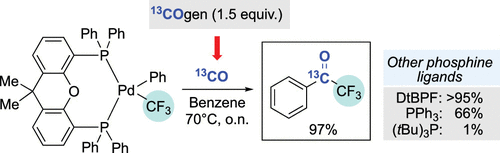
We have performed a series of stoichiometric studies in order to identify viable steps for a hypothetical catalytic cycle for the palladium-mediated carbonylative coupling of an aryl bromide with TMSCF3. Our work revealed that benzoyl Pd(II) complexes bearing Xantphos or tBu3P as the phosphine ligands, which are generated from the corresponding PdII(Ph)Br complexes exposed to stoichiometric 13CO from 13COgen, were unable to undergo transmetalation and reductive elimination to trifluoroacetophenone. Instead, in the presence of base and additional CO, these organometallic complexes readily underwent reductive elimination to the acid fluoride. Attempts to determine whether the acid fluoride could represent an intermediate for acetophenone production were unrewarding. Only in the presence of a boronic ester did we observe some formation of the desired product, although the efficiency of transformation was still low. Finally, we investigated the reactivity of four phosphine-ligated PdII(Ph)CF3 complexes (Xantphos, DtBPF, tBu3P, and triphenylphosphine) with carbon monoxide. With the exception of the tBu3P-ligated complex, all other metal complexes led to the facile formation of trifluoroacetophenone. We also determined in the case of triphenylphosphine that CO insertion occurred into the Pd–Ar bond, as trapping of this complex with n-hexylamine led to the formation of n-hexylbenzamide.
中文翻译:

以TMSCF 3为三氟甲基源的芳基Pd(II)配合物羰基化三氟甲基化的化学计量研究
我们已经进行了一系列的化学计量研究,以确定可行的步骤为一个假设的催化循环的芳族溴化物与TMSCF 3的钯介导的羰基化偶联。我们的工作表明,带有Xantphos或t Bu 3 P作为膦配体的苯甲酰基Pd(II)配合物是由相应的Pd II(Ph)Br配合物从13暴露于化学计量的13 CO中生成的二氧化碳不能进行金属转移和还原消除为三氟苯乙酮。相反,在碱和另外的CO的存在下,这些有机金属配合物容易进行还原性消除为酰氟。试图确定酸性氟化物是否可以代表苯乙酮生产的中间体的尝试是没有意义的。尽管转化效率仍然很低,但只有在硼酸酯存在下,我们才能观察到所需产物的某些形成。最后,我们研究了四种膦连接的Pd II(Ph)CF 3配合物(Xantphos,D t BPF,t Bu 3 P和三苯膦)与一氧化碳的反应性。除了t Bu 3 P连接的配合物,所有其他金属配合物导致三氟乙酰苯的容易形成。我们在三苯基膦的情况下,也判定为CO插入发生到的Pd-Ar键,与该复合物的捕获Ñ己胺导致形成Ñ -hexylbenzamide。
更新日期:2020-02-18
中文翻译:

以TMSCF 3为三氟甲基源的芳基Pd(II)配合物羰基化三氟甲基化的化学计量研究
我们已经进行了一系列的化学计量研究,以确定可行的步骤为一个假设的催化循环的芳族溴化物与TMSCF 3的钯介导的羰基化偶联。我们的工作表明,带有Xantphos或t Bu 3 P作为膦配体的苯甲酰基Pd(II)配合物是由相应的Pd II(Ph)Br配合物从13暴露于化学计量的13 CO中生成的二氧化碳不能进行金属转移和还原消除为三氟苯乙酮。相反,在碱和另外的CO的存在下,这些有机金属配合物容易进行还原性消除为酰氟。试图确定酸性氟化物是否可以代表苯乙酮生产的中间体的尝试是没有意义的。尽管转化效率仍然很低,但只有在硼酸酯存在下,我们才能观察到所需产物的某些形成。最后,我们研究了四种膦连接的Pd II(Ph)CF 3配合物(Xantphos,D t BPF,t Bu 3 P和三苯膦)与一氧化碳的反应性。除了t Bu 3 P连接的配合物,所有其他金属配合物导致三氟乙酰苯的容易形成。我们在三苯基膦的情况下,也判定为CO插入发生到的Pd-Ar键,与该复合物的捕获Ñ己胺导致形成Ñ -hexylbenzamide。



























 京公网安备 11010802027423号
京公网安备 11010802027423号